Genetic transformation techniques- Gene gun mediated, Agrobacterium mediated transformation - Overexpression, antisense expression (in model as well as crop plants)
Theory
Agrobacterium-mediated transformation is a technique used to introduce new genes into plants. Agrobacterium tumefaciens is a soil bacterium that causes a plant disease known as crown gall. This disease affects plants such as roses, grapes, and others by causing tumour-like growths called galls on the stems or at the crown (the base where the plant meets the soil). The unique feature of Agrobacterium is its ability to naturally insert its DNA into plant cells, earning it the title "natural plant genetic engineer."
Agrobacterium transfers a specific piece of DNA, called T-DNA, from its own genome into the plant's DNA. The T-DNA is part of a larger structure called the Ti plasmid, which originally caused tumours in plants. Scientists have modified the Ti plasmid to remove the tumour-causing ability but still allow the T-DNA to transfer into plants. This method has become the preferred way of introducing new genes into plants because it is simpler and less expensive than other techniques, such as direct DNA transfer methods that require costly equipment.
Agrobacterium is easy to grow and can be applied directly to plant tissues, making the process more convenient. Unlike direct DNA delivery methods, where DNA is forced into the plant cell's nucleus, the T-DNA from Agrobacterium naturally includes signals that help it reach the nucleus on its own.
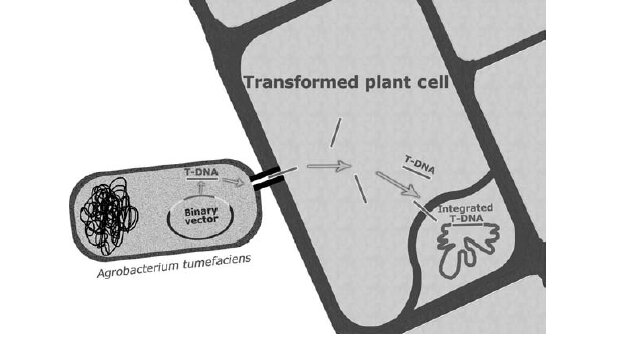
Figure 1. Schematic of Agrobacterium-mediated transformation of a plant cell, showing production of the T strand from the binary vector, transport through the bacterial pillus, and integration into plant chromosomal DNA.
Since the 1980s, scientists have improved our understanding of how Agrobacterium transfers DNA, making it more efficient for plant genetic engineering. One interesting fact about crown gall disease is that the tumours keep growing even after the bacteria are no longer present. The disease occurs when Agrobacterium infects wounded plant tissues, especially at the crown. The bacteria attach to the damaged cells and send chemical signals, triggering the transfer of T-DNA into the plant's genome. This T-DNA contains genes that make the plant produce hormones and compounds called opines, which are nutrients for the bacteria.
The plant hormones cause tumour growth, while the opines feed the bacteria. The bacteria don't live inside the tumour cells but instead use the opines that the tumour cells release. Tumours can continue to grow in a laboratory setting without added hormones, showing that the tumours are independent of the bacteria.
Research has shown that altering the hormone-producing genes in Agrobacterium changes the type of tumour that forms. Disrupting one hormone gene causes the plant to grow abnormal roots, while disrupting another leads to abnormal shoots. If both genes are disrupted, no tumour forms. This research has helped scientists understand the role of hormones in plant tumour growth.
Factors Affecting Agrobacterium-Mediated Gene Transfer
Several methods are used to create genetically modified plants, including Agrobacterium-mediated transformation, particle bombardment, protoplasts via polyethylene glycol, and liposome-mediated transformation. Among these, Agrobacterium-mediated transformation tends to produce more stable single-copy transgenes compared to multiple gene copies. However, the efficiency of this process depends on various factors such as bacterial strains, cell density, plant species, plant growth regulators, and environmental conditions. Finding the right balance of these factors is essential for developing an effective transformation protocol.
Key factors influencing Agrobacterium-mediated transformation include:
- Explants: Explants can include embryonic cultures, immature embryos, mature seed derivatives, leaf blades, or stem pieces. Selecting suitable explants that support the regeneration of the transgenic plant is crucial, with embryonic callus from mature seeds often being ideal. Studies suggest that drying explants before or after Agrobacterium infection improves transformation efficiency.
- Explant Wounding: Wounding the explants enhances transformation success. Methods range from simple cuts to particle gun-mediated micro-wounding, sonication, and Agrobacterium-loaded syringes. These wounds release phenolic compounds that aid in transformation.
- Plant Species and Genotype: Transformation efficiency varies across plant species and genotypes due to differences in vir gene expression and chromosomal/plasmid genome factors. Dicots are generally more responsive to Agrobacterium transformation, though gene transfer in monocots has become more common.
- Antibiotics: Post-transformation, antibiotics like carbenicillin and cefotaxime are used to eliminate bacteria without harming the host plant. The choice and concentration of antibiotics depend on the plant and Agrobacterium strain.
- Plant Growth Regulators (PGR): PGRs are essential for transformation, with 2,4-D commonly enhancing efficiency. They help promote cell division and differentiation but should be applied at specific stages of the plant cell cycle.
- Light: Light influences transformation by affecting hormone levels, cell proliferation, and vir gene induction. Dark co-cultivation conditions are often used to improve explant responses, and photoperiod can be a significant factor.
- Temperature: High temperatures (around 32°C) inhibit tumour formation, while a range of 19°C to 22°C is optimal for T-DNA transfer in many plant species.
- Agrobacterium Strains: Different Agrobacterium strains carry plasmids that affect their infection capabilities. A combination of super-virulent strains with suitable binary vectors often produces the best results.
Principle of Agrobacterium-mediated Gene Transfer
- Agrobacterium-mediated transformation relies on the efficient transfer of T-DNA from the bacterium to host plant cells.
- This process involves two key regions: the T-DNA, which contains 25-bp direct repeats, and the virulence (vir) region with seven main loci.
- During transformation, a portion of the plasmid from the bacterium is transferred into the plant cells and integrates into the nuclear genome, altering hormone levels to favour bacterial survival.
- The bacteria also produce enzymes to synthesize opines, which serve as their food source.
- Key components include the tumour-inducing (Ti) plasmid, which carries T-DNA, and other functional regions like virulence (vir), conjugation (con), and the origin of replication (ori).
- Infection begins when the bacterium enters through plant wounds, releasing acetosyringone (AS), which helps bind the bacteria to the plant cells.
- AS activates the bacterial VirA proteins, which then activate VirG through phosphorylation. VirG, in turn, induces other vir genes, leading to T-strand production.
- The VirD2 protein attaches to the 5' end of the T-strand, forming a T-complex with other proteins like VirE2 and VirB, which guide the T-DNA into the plant nucleus.
- The integration of T-DNA into the plant genome occurs through recombination, usually in transcriptionally active or repetitive regions.
- While much is known about T-DNA transfer at the molecular level in Agrobacterium, the plant factors involved in the process remain less understood.
Applications of Agrobacterium-mediated Gene Transfer
- This method has been used to engineer plants to produce proteins, antibodies, and pharmaceuticals, such as anticoagulants and growth factors.
- Transgenic plants serve as biomonitors for detecting pollutants and cleaning contaminated soil and water.
- Agrobacterium-mediated transformation has improved crop yields by enhancing food storage and production.
- It enables the development of crops with enhanced resistance to biotic and abiotic stresses, nutrient uptake, and pest resistance.
- Genes like Bt toxin have been introduced to create insect-resistant crops, reducing the need for harmful pesticides.
- While primarily used for plants, this method shows potential for broader applications in genetic engineering.
Limitations of Agrobacterium-mediated Gene Transfer
- This method is limited to certain plant species.
- Though T-DNA transfer is well-understood in bacteria, less is known about plant-expressed factors that influence its success.
- The process is labour-intensive, requiring complex regeneration protocols and lengthy steps, with in vitro variations leading to negative effects.
- In monocots, transformation success depends on using embryos, which are available only seasonally.
- Agrobacterium-mediated transformation struggles to transfer large DNA molecules into economically significant plants, suggesting a need for more powerful vector systems.
Use of the T-DNA Transfer Process for Transformation
The process of using Agrobacterium to insert genes into plants like wheat and corn has greatly improved. This progress is due to a better understanding of how Agrobacterium transfers its DNA and interacts with plant cells. A simplified process is given below:
- Basic Setup:
- Agrobacterium uses a plasmid to transfer genes to plants. Scientists now use a simplified version with just the essential components for gene transfer.
- Two plasmids are involved: a modified Ti plasmid that aids the transfer, and a binary vector containing the T-DNA with the desired genes.
- Plasmid Preparation:
- The binary vector carries the T-DNA region without the genes for hormones and opines, and the modified Ti plasmid has the machinery needed to process and transfer the T-DNA.
- Infection Process:
- When placed on wounded plant tissue, Agrobacterium is activated by the plant’s release of acetosyringone, triggering virulence genes (vir genes). To overcome difficulties with plants like wheat and corn, synthetic acetosyringone is added.
- DNA Transfer:
- Once the vir genes are active, the T-DNA is prepared and transferred into the plant cell via a pilus, a tube that connects the bacterium to the plant cell.
- Integration into Plant DNA:
- The T-DNA moves into the plant cell's nucleus, integrating into the plant’s genome, typically in active regions of the DNA.
Optimizing Gene Delivery: Scientists are improving Agrobacterium's efficiency by focusing on:
- Enhancing Delivery: Precise methods like sonication or particle bombardment create small wounds for bacteria entry without damaging the plant.
- Activating vir Genes: Adding acetosyringone or using modified bacteria with always-active vir genes improves the transformation process.
- Reducing Plant Defense: Chemicals like cysteine or ascorbic acid reduce oxidation and browning, enhancing the transformation efficiency.
Agroinfiltration: This method assists in a quick study of gene expression without creating a fully transformed plant. Agrobacterium is injected into plant leaves (e.g., Nicotiana benthamiana), and viral genes can be added to amplify the introduced gene’s effect. Agroinfiltration is fast, allowing scientists to assess gene effects without growing a whole plant.
Arabidopsis Floral Dip: Arabidopsis is a model plant for genetic research due to its small size, fast growth, and ease of transformation. The floral dip method, where flowering plants are dipped in an Agrobacterium solution, introduces new genes that are passed down through seeds. While this method works well for Arabidopsis, it is less effective for plants like corn or soybeans, which remain a challenge for scientists.
In summary, improvements in Agrobacterium-mediated gene transfer have streamlined the process for many plants, though challenges remain for certain crops like corn and soybeans.
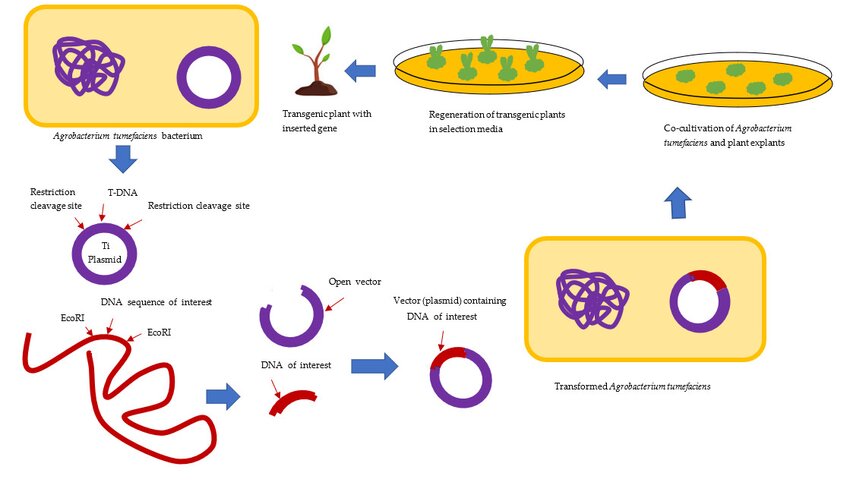
Figure 2. Agrobacterium-Mediated Gene Transfer (Transformation) in Plants
