X-Ray Fluoresce microscopy for determination of chemical composition of materials
X-rays fluorescence (XRF) spectroscopy techniques is used for determination of elements (quantitative and qualitative) of materials (metals, alloys and ceramics). It gives the comprehensive details from ppm to high percentage of elemental composition of sample.
The fundamental of XRF is based on emission of secondary (Fluorescent) X-rays from a sample that has been irradiated by primary beam of electrons or X-rays photons. Atom which consists of a nucleus and electron in orbitals (K, L, M Shells). As primary source (X-rays or electrons) from X-ray tube falls on atoms, present in the sample, the electrons from inner shell got knock off and e-vacancy is created as shown in Fig 1. The atom is in excited state lower its energy by filling the inner shell e-vacancy from outer shells electrons and energy is released in the form of secondary x-rays or fluorescence. Each element in the sample produces characteristic X-rays which provide elemental determination of sample. The intensity of x-rays provides the quantitative mapping of elements whereas characteristic energy levels provide the qualitative mapping of elements. By analysing the positions and intensities of the peaks, we can identify and quantify the elements present in the material.
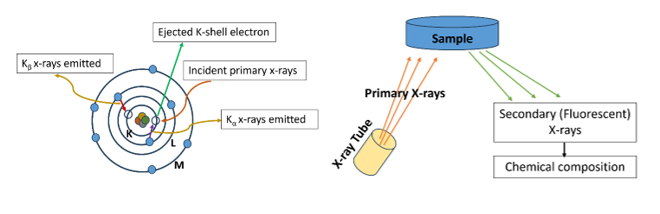
Fig 1 : Generation of secondary x-rays from primary source
