X-Ray Fluoresce microscopy for determination of chemical composition of materials
Methodology:
Sample Irradiation and Initial Beam Formation :
a. The sample is irradiated with an X-ray source.
b. The emitted X-ray fluorescence from the sample is directed towards collimators.Collimation :
a. Collimators are used to control the divergence of the X-ray beam, ensuring a parallel or near-parallel beam.
b. The collimated X-ray beam is directed onto an analyzing crystal.
c. A selection of different crystals (e.g., LiF, Ge, pentaerythritol, thallium acid phthalate) as mentioned in Table 1, is used to cover a wide range of X-ray wavelengths.
d. Crystal fluorescence is also used for background noise reduction.
e. The analyzing crystal disperses the X-rays according to Bragg's Law, separating them into their constituent wavelengths.Diffracted Beam Detection and result :
a. A detector receives the diffracted X-ray beam from the analyzing crystal.
b. The detector converts the received X-ray radiation into measurable voltage pulses.
c. The voltage pulses are processed and converted into a pulse height distribution (PHD) plot, which represents the intensity of X-rays at different energies as shown in Fig 2.
d. Identification of X-rays peaks for the determination of chemical composition of materials and the intensity of peaks provide the information about the concentration of composition of material.
Table 1: List of crystals used for detection of wide variety of materials (metals, alloys and ceramics)
| Crystals used for analyzing crystal | Energy of X-rays |
|---|---|
| LiF (Lithium fluoride) | Very short to medium energy (nickel, copper, Zinc) |
| Ge (Germanium) | For higher energy x-rays (titanium, vanadium, etc.) |
| PE (Pentaerythritol) | For medium energy x-rays (chlorine, sulfur, phosphorus) |
| TIAP (Thallium acid phthalate) | For light energy x-rays (oxygen, carbon, nitrogen) |
| Crystal Fluorescence | For background noises |
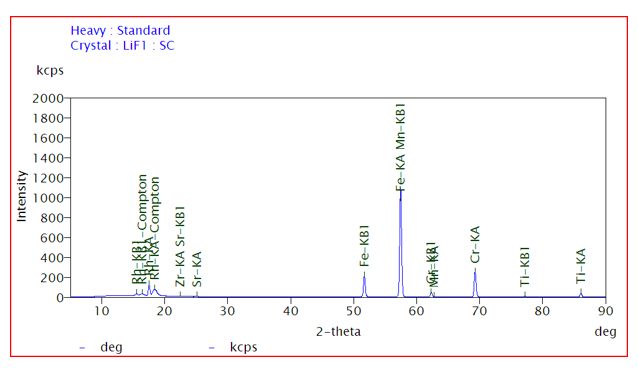
Fig 2: Elemental mapping of sample (intensity vs energy)
