Analyze quality and quantity of fats
Theory
Introduction to Lipids
Lipids are a diverse group of naturally occurring organic compounds that are insoluble in water but soluble in non-polar solvents such as chloroform, ether, or benzene. They include fats, oils, waxes, phospholipids, and steroids, which play vital roles in biological systems — serving as structural components of cell membranes, energy reserves, and precursors for hormones and signaling molecules.
Chemically, lipids are composed mainly of carbon, hydrogen, and oxygen, and often contain other elements such as phosphorus or nitrogen. Most natural fats and oils are triesters of glycerol and fatty acids, known as triglycerides. The nature and number of fatty acid chains, especially the presence or absence of double bonds, determine whether a lipid is saturated or unsaturated, and influence its physical properties and nutritional quality.
The biochemical tests described in this experiment help to identify and characterize lipids based on their physical and chemical properties. These include qualitative tests (such as Acrolein and Sudan IV tests) for detection, and quantitative tests (such as Saponification, Acid, and Iodine values) that assess the composition, purity, and quality of lipid samples. Together, these experiments lay the foundation for understanding lipid chemistry and its applications in food, health, and industrial contexts.
Acrolein test:
The Acrolein test is used to detect the presence of glycerol, which confirms the presence of fats. This test is based on the dehydration reaction of glycerol in the presence of potassium hydrogen sulphate (KHSO4). Glycerol loses water molecules and forms acrolein, which has a characteristic pungent and irritating odor.
C3H5(OH)3 → CH2= CH-CHO + 2H2O
Inference: The appearance of a pungent smell confirms the presence of glycerol and hence lipids.
Sudan IV Test
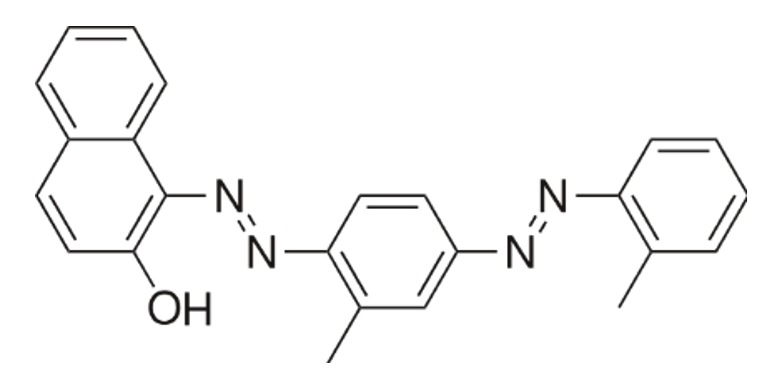
Common name: Sudan IV, Chemical class: Azo dye, Molecular formula: C24H20N4O
The dye has Azo Linkage (-N=N-), the core structural feature of Sudan IV, connects two aromatic ring systems, and they are responsible for strong coloration (deep red) and light absorption in the visible range
The Sudan IV test is used to detect the presence of lipids in a sample. It is based on the principle that Sudan IV is a non-polar dye and dissolves preferentially in non-polar substances such as fats and oils. When added to a lipid-containing sample, it stains the lipid red-orange, while polar substances remain unstained.
Inference: Development of red-orange coloration confirms the presence of lipids.
Furfural and Baudouin Test:
These tests are specifically used to detect adulteration in ghee.
Furfural Test: Detects the presence of sesame oil.
Baudouin Test: Detects hydrogenated vegetable oils (vanaspati).
In the presence of concentrated hydrochloric acid and furfural, sesame oil produces a crimson red color due to the reaction of sesamol with furfural.
Inference: Appearance of crimson red color confirms adulteration.
Huble's Test:
This test is used to determine the degree of unsaturation in oils and fats. Unsaturated fatty acids contain carbon–carbon double bonds which react with iodine. The more the unsaturation, the greater is the uptake of iodine.
R-CH=CH-R + I2 → R-CHI-CHI-R
During the test, iodine solution is added to the lipid dissolved in chloroform until the pink color persists. The amount of iodine absorbed indicates the level of unsaturation.
Saponification value:
The saponification value is defined as the number of milligrams (mg) of potassium hydroxide (KOH) required to completely saponify 1 gram of oil or fat.
Unit: mg KOH g-1 of oil
C3H5(C7H35COO)3 + 3KOH = C3H5(OH)3 + 3C17H35COOK
(Triglyceride → Glycerol + Potassium stearate (soap))
Lower molecular weight fatty acids require more alkali; therefore, the saponification value is inversely proportional to the molecular weight of fatty acids. Higher saponification values indicate the presence of short- and medium-chain fatty acids and higher soap-forming ability.
Formula: Saponification value (S.V)= 56.1xMx (Vo-V1)/m
- M = Molarity of hydrochloric acid (HCl)
- Vo: Volume of HCl for blank (cm3)
- V1: Volume of HCl for sample (cm3)
- m = Mass of oil (g)
Acid Value:
The acid value is defined as the number of milligrams (mg) of potassium hydroxide (KOH) required to neutralize the free fatty acids present in 1 gram of fat or oil.
Unit: mg KOH g-1 of oil
The sample is dissolved in a solvent mixture and titrated with 0.1 N potassium hydroxide (KOH) using phenolphthalein as an indicator.
Free fatty acids are formed due to hydrolysis and oxidation, leading to rancidity. Oils with high acid value cause corrosion of metal containers and are unsuitable for consumption.
Iodine Value
The iodine value is defined as the number of grams of iodine absorbed by 100 grams of oil or fat under specified conditions.
Unit: g I2 per 100g oil
It is a direct measure of the degree of unsaturation. Higher iodine values indicate higher unsaturation, which is desirable in drying oils but undesirable for edible oils due to oxidative instability.
Real-World Applications of Lipid Analysis
Understanding the chemical behavior of fats and oils has wide-ranging real-world applications in fields such as nutrition, food technology, biochemistry, and public health.
- Food Quality and Adulteration:
- Tests like the Furfural and Baudouin tests are routinely used to detect adulteration in ghee and edible oils, ensuring authenticity and consumer safety.
- Industrial and Commercial Applications:
- Saponification value helps determine the chain length of fatty acids, which is essential in selecting oils for soap manufacturing, cosmetics, and lubricants.
- Iodine value guides industries in assessing oil unsaturation, stability, and suitability for products like margarine or biodiesel.
- Health and Nutritional Assessment:
- Acid value acts as an indicator of rancidity, reflecting the oxidative degradation of fats — crucial in evaluating shelf life and safety of food products.
- In biomedical contexts, lipid analysis contributes to understanding metabolic disorders, obesity, and cardiovascular diseases.
- Research and Education:
- These tests provide hands-on experience linking chemical principles to biological materials, bridging theoretical learning with practical biochemical analysis.
