Detection and estimation of oxidative rancidity in fats/oils
In oxidative rancidity oxygen is taken up by the fat with the formation of peroxides. The peroxide value is a useful indicator of the extent of oxidation of lipids, fats, and oils. The degree of peroxide formation and the time taken for the development of rancidity differ among oils. Time, temperature, light, air, exposed surface, moisture, and traces of metals are the major factors responsible for rancidity. The oxidation of food lipids is undesirable due to off-flavors, toxins, and loss of fat-soluble vitamins. In addition, the analysis of the peroxide content of oil samples is an important analytical task because high peroxide levels in oils have been a threat to human health.
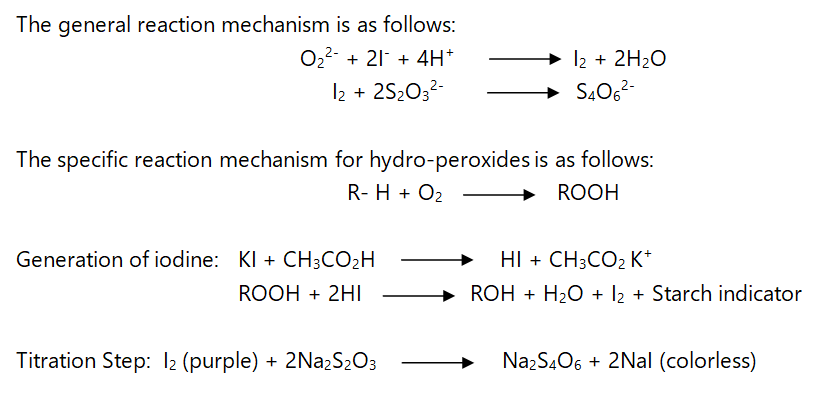
Peroxide value is defined as the milliequivalents of peroxide per kilogram of fat, as determined in a titration procedure to measure the amount of peroxide or hydroperoxide groups. The peroxides present are determined by titration against thiosulphate in the presence of KI. Starch is used as indicator. In general fresh oils have a peroxide value of <10 mEq/Kg while peroxide values in the range of 30-40 mEq/Kg are generally associated with a rancid taste.
The general reaction mechanism is as follows: