1. Determination of heat stability of vitamin C
Materials required
- Analytical balance
- Beaker
- Burette
- Conical flasks
- Measuring cylinders
- Ascorbic acid standard solution
- DCPIP dye solution
- Metaphosphoric acid solution
- Juice samples- Apple, Mousami, Lemon and Amla
Methods
Standardization of Dye
- Arrange all the prepared reagent solutions and test samples on the table.
- Add 5 ml ascorbic acid standard solution to each flask.
- Add 5 ml of HPO3 solution in the conical flask
- Fill the burette with the DCPIP dye solution and record the initial burette reading.
- Place the conical flask under the tip of the burette.
- Slowly add DCPIP dye solution to standard ascorbic acid solution until pink color appears which persists for 15s.
- Record final burette reading and calculate the dye factor using this formula:
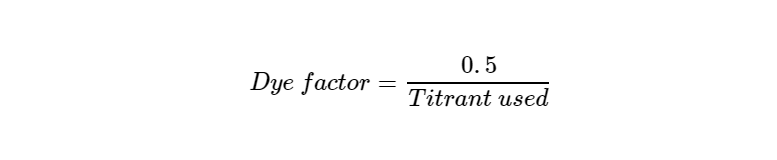
Analysis of Juice Samples
- Select the sample and take out 50 ml juice in four different conical flasks marked as J1, J2, J3, J4.
- Heat the J2, J3, J4 sample at 60°C, 75°C and 90°C, respectively,
- Pipette out 10 ml juice from each sample flask and pour to the four different conical flask
- Add 90 ml of 3% HPO3 solution in each conical flask
- Titrate each sample with DCPIP dye solution until pink color appears which persists for 15s.
- Record the initial and final readings to get the amount of dye used for each titration.
- Calculate the Ascorbic acid (mg/100gm) using the following equation:

