Surface Tension of Kerosene by Drop Weight Method
Theory :
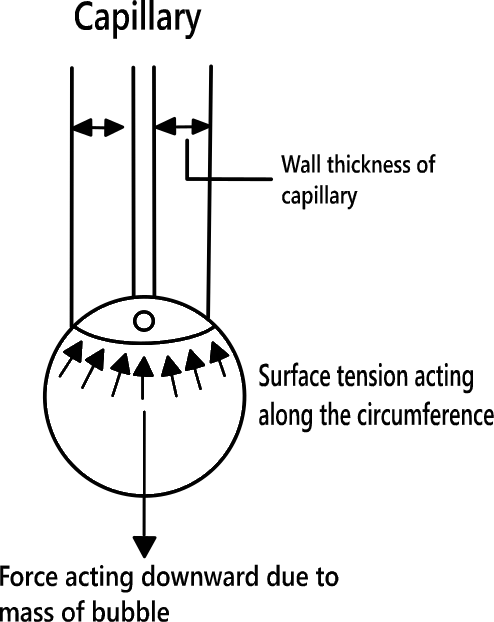
This method is based on the principle that the weight of a drop falling slowly out of a capillary held vertically is directionally proportional to its surface tension. The drop falls down when its weight just exceeds surface tension. When the drop just breaks away, the force pulling it upwards is:
2πrγ
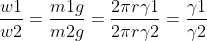
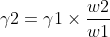
Where,
r = Radius of capillary.
γ = surface tension.

At equilibrium: 2πrγ= mg
For two liquids the drops of which fall almost at the same rate out of the same capillary
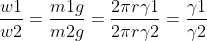
Surface tension of required solution
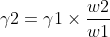
Where, W1 and W2 => Weights of liquids
γ1 and γ2 => the surface tension of liquids
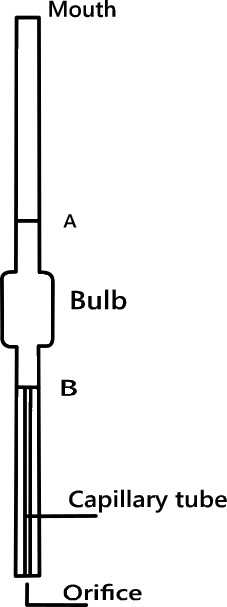
The apparatus used for determining surface tension of a liquid is known as stalagmometer. It resembles a pipette with a bulb in the middle and a mark A on the tube above the bulb. The lower portion is made up of a capillary with a flattened tip.

