Thin layer chromatography of ink
Chromatography is extensively used by the chemists to separate compounds in a mixture.
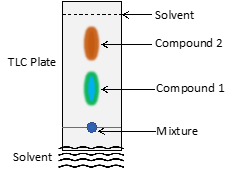
Although many types of chromatography have been developed, all methods use a mobile phase and a stationary phase. In this experiment, we will be using thin layer chromatography, or TLC. In TLC, a mobile phase (i.e. solvent) migrates up a thin layer of solid particles attached to a glass plate (called the stationary phase). Chromatography works because of the differences in polarity between the stationary phase, the solvent, and the components of the mixture being studied. When using a solvent with low polarity, the majority of the components of the mixture, if not all of them, will be held more strongly to the stationary phase than the solvent molecules, so the solvent will not displace them and carry them along with it. If a more polar solvent is chosen, it will displace the majority of the molecules in the sample. Depending on the polarity of each of the components, some may travel farther with the solvent than others, resulting in separation of each of the components (see Fig 1).
The solvent and solute travel certain distances along the glass plate and these distances are used to calculate the Rf value as:
Rf =(Distance travelled by the solute)/(Distance travelled by the solvent)
Different components of ink have own characteristic Rf values in the solvent and this make identification possible. The choice of solvent is important for utility of chromatography. A solvent that is much more polar than the components of the mixture will displace all of the molecules and carry them substantially on the solvent front, yielding poor separation that would not be useful in identification of components.
