Synthesis of Aspirin
Glassware required :
- Weighing balance
- Conical flask (150 ml)
- Measuring cylinder (10 ml, 100 ml)
- Glass dropper
- Filtering flask
- Water bath
- Ice bath
Chemicals required :
- Salicylic acid
- Acetic Anhydride
- Concentrated Sulfuric Acid
- Ethanol
- Distilled water.
Laboratory procedure :
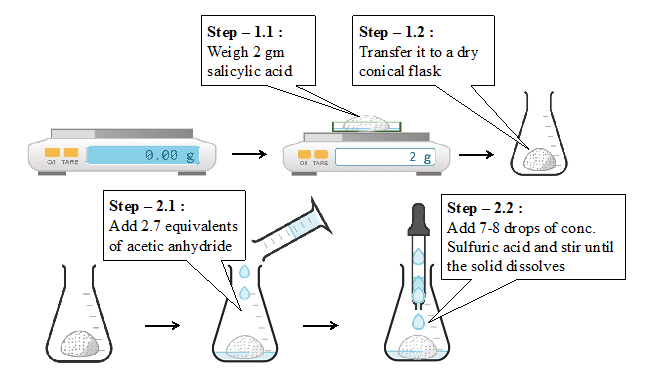
- Weigh 2.0 g of salicylic acid (Mol. Wt. 138.12 g/mol) in a watch glass and transfer it to a dry 150 ml conical flask.
- Add 2.7 equivalents of acetic anhydride (Mol. Wt. 102.08 g/mol) using a measuring cylinder to the salicylic acid. Add 7-8 drops of concentrated sulfuric acid and stir until all salicylic acid is dissolved.
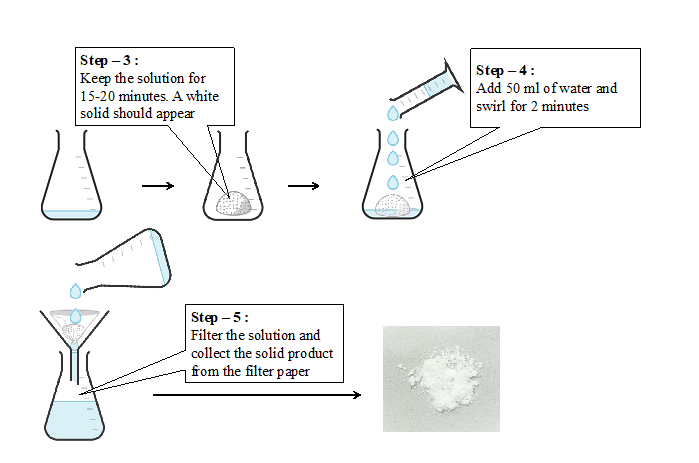
- Leave the reaction mixture undisturbed for 20 minutes. A white solid should appear.
- Add 50 ml water to the flask and swirl for two minutes and filter.
- Collect the solid from the filter paper.
Recrystallization :
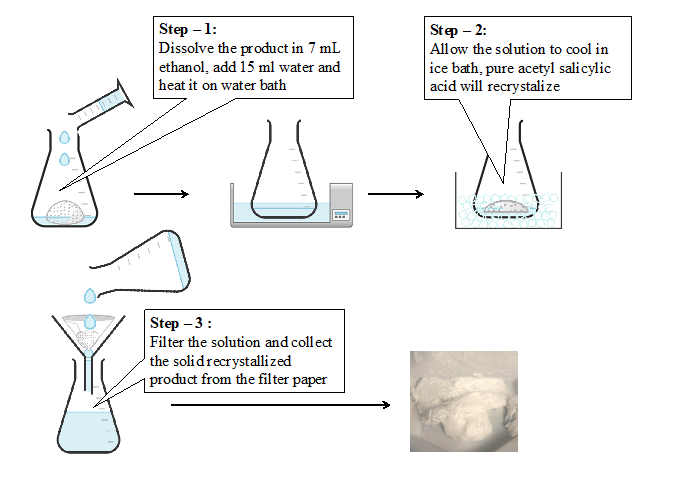
- Dissolve the crude product in 7 ml ethanol in a beaker and add 15 ml of distilled water. Heat on water bath till you get a clear solution.
- Allow the solution to cool in an ice bath without disturbing. Pure acetylsalicylic acid crystalizes.
- Filter the pure product and allow it to dry.
Plausible mechanism :
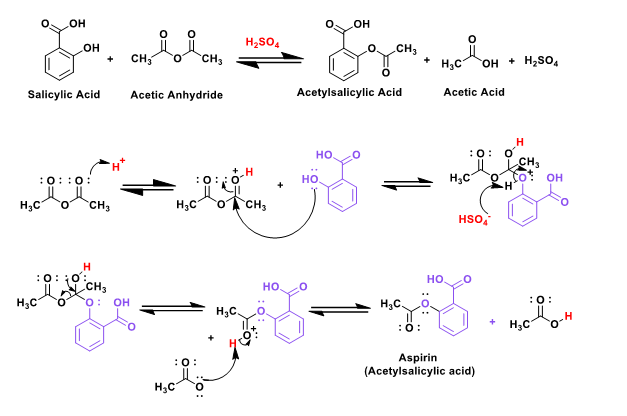
Laboratory procedure (diagram) :
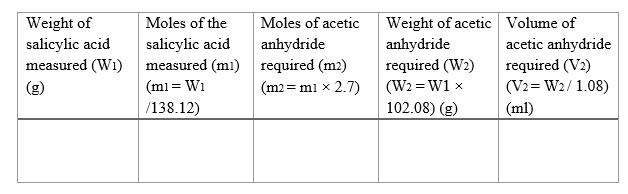
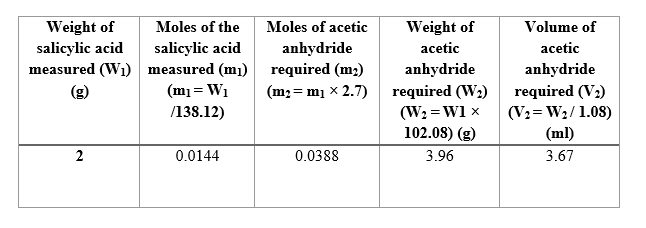
Calculate the required amount of acetic anhydride-
Recrystallization (diagram) :
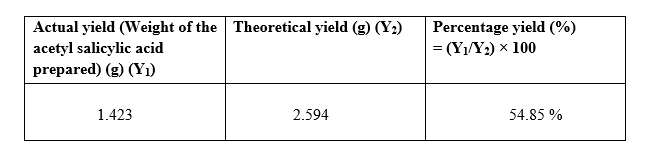
Yield of the reaction :

Calculation for the amount of acetic anhydride needed for a given amount of salicylic acid :
We have been asked to add 2.7 equivalents of acetic anhydride with respect to salicylic acid.
Mol. Wt. of salicylic acid = 138.12 g/mol
Mol. Wt. of acetic anhydride = 102.08 g/mol
Density of acetic anhydride = 1.08 g/ml
Calculation of percentage yield of the prepared Aspirin :
Mol. Wt. of Aspirin = 180.158 g/mol
From the chemical equation we know that 1 mole of salicylic acid will give 1 mol of acetyl salicylic acid / Aspirin.
Calculation of theoretical yield of acetyl salicylic acid:
Moles of salicylic acid taken = 0.0144 moles
Hence, we should get 0.0144 moles of acetyl salicylic acid
Theoretical yield = 0.0144 moles = 2.594 grams of acetyl salicylic acid.
Percentage yield :