Kinetics of iodide-hydrogen peroxide clock reaction
Materials & Reagents Required:
- Conical Fluxes (250 mL)
- Measuring Cylinder (25 mL)
- Volumetric Fluxes (100mL)
- Stopwatch
- Glass Rod
Procedure in laboratory (diagram)
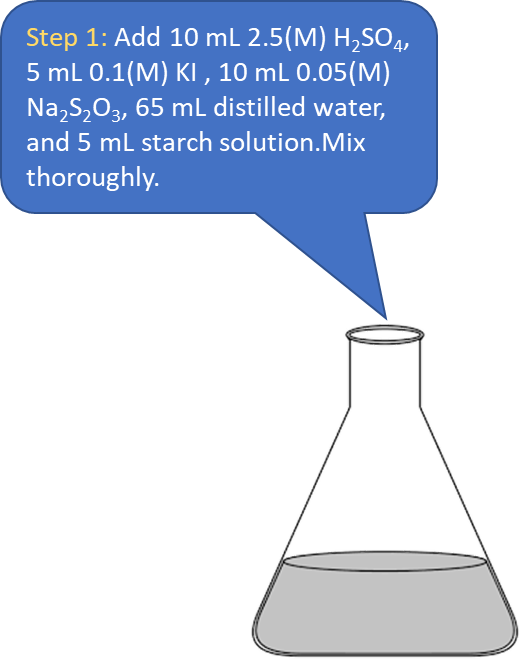
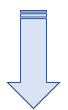
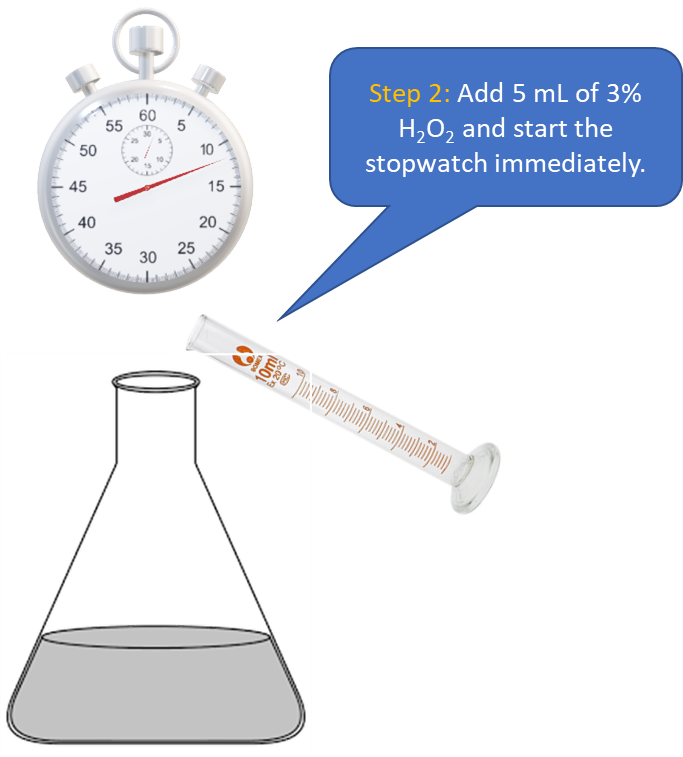

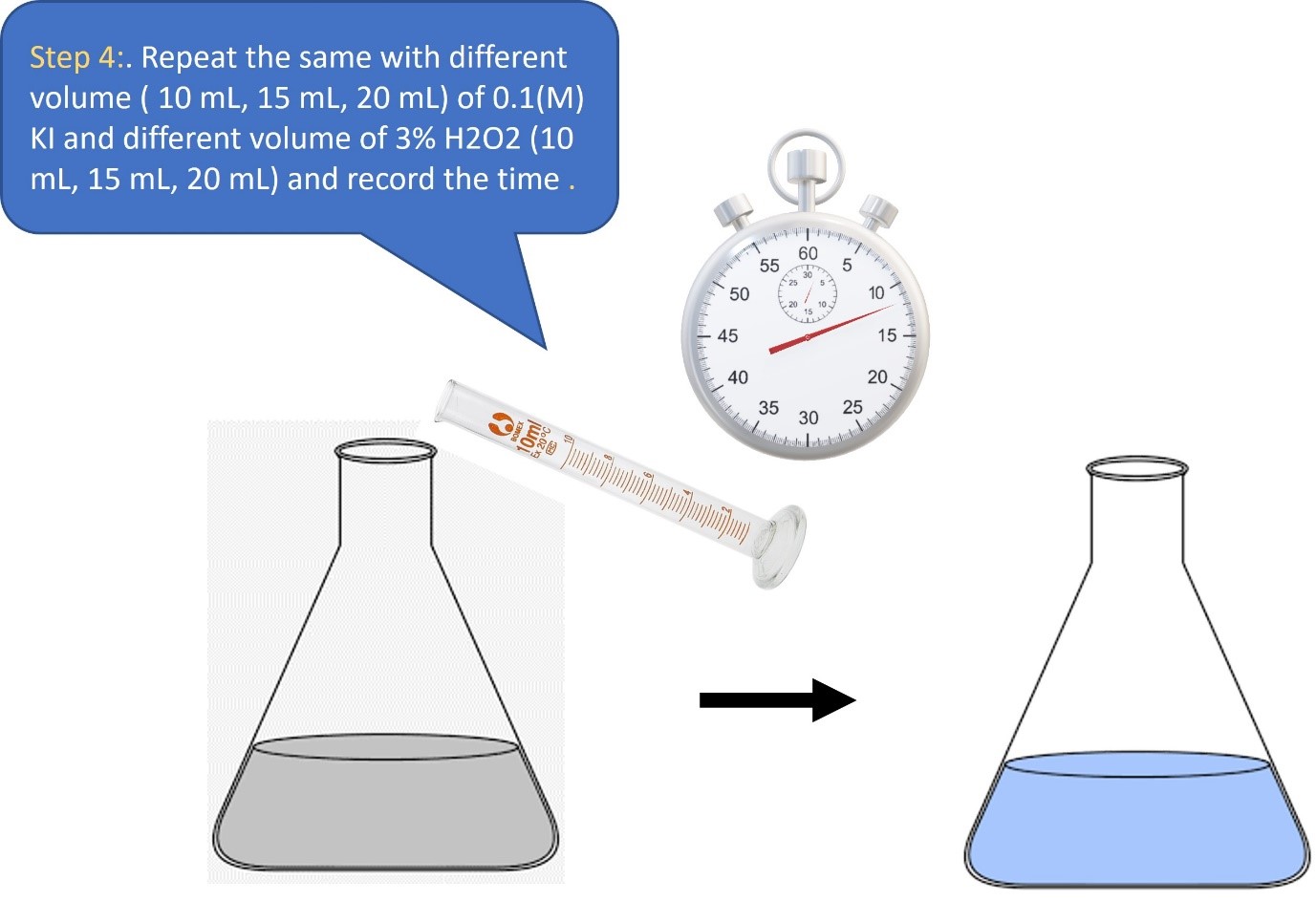


Procedure in laboratory

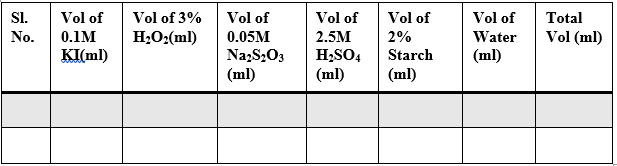
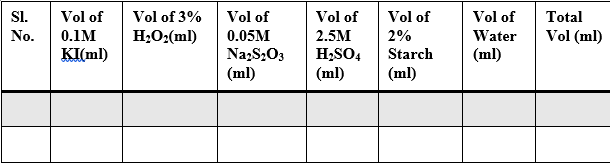
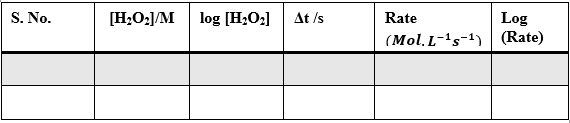
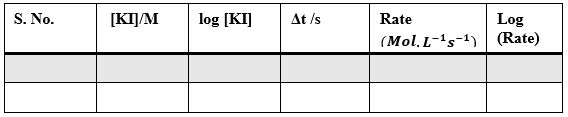
Sample Data and Analysis
Table – 1
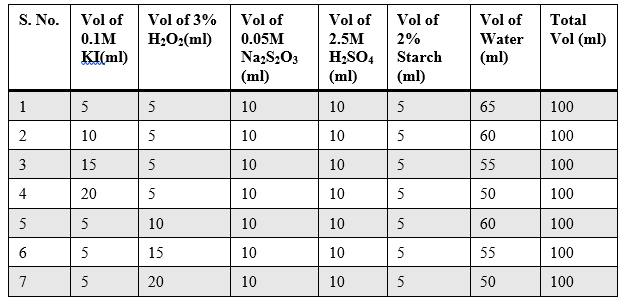
Table – 2
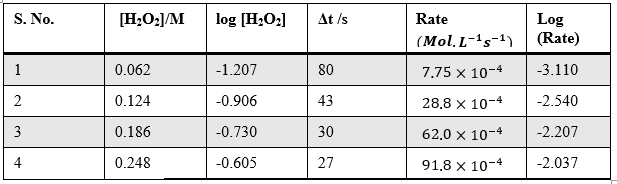
Table – 3
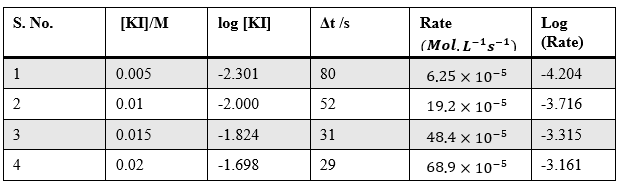
Analysis
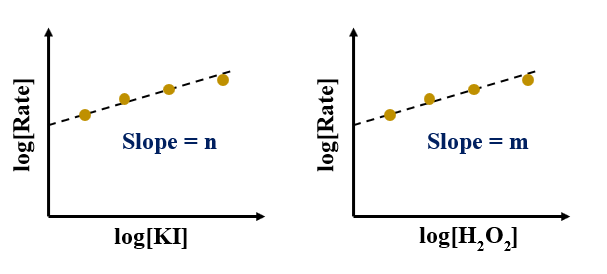
Plot of log[Rate] vs log[H2O2]:
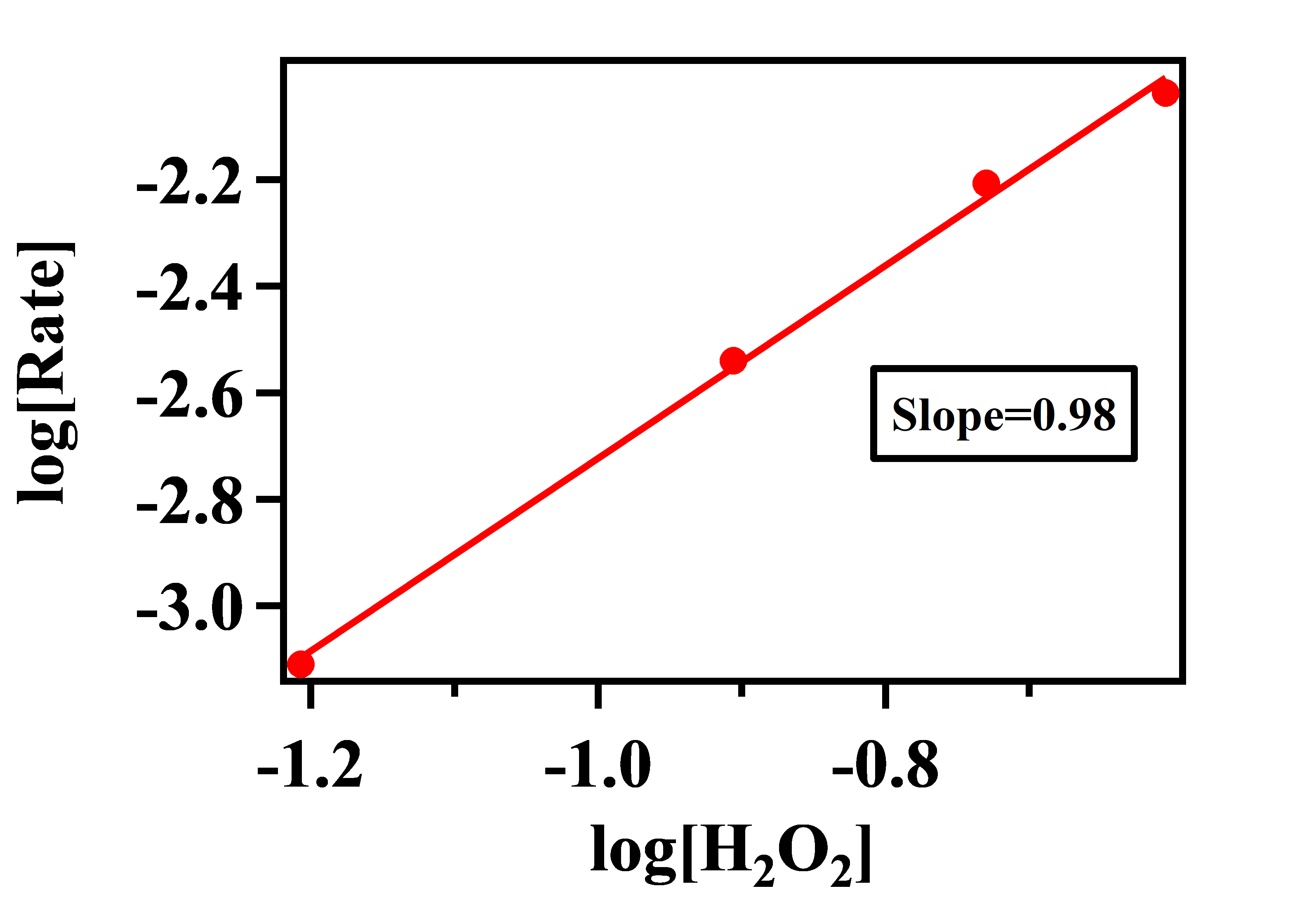
Here, the order of the reaction with respect to H2O2 = 1 (nearest possible integer of 0.98)
Plot of log[Rate] vs log[KI]:
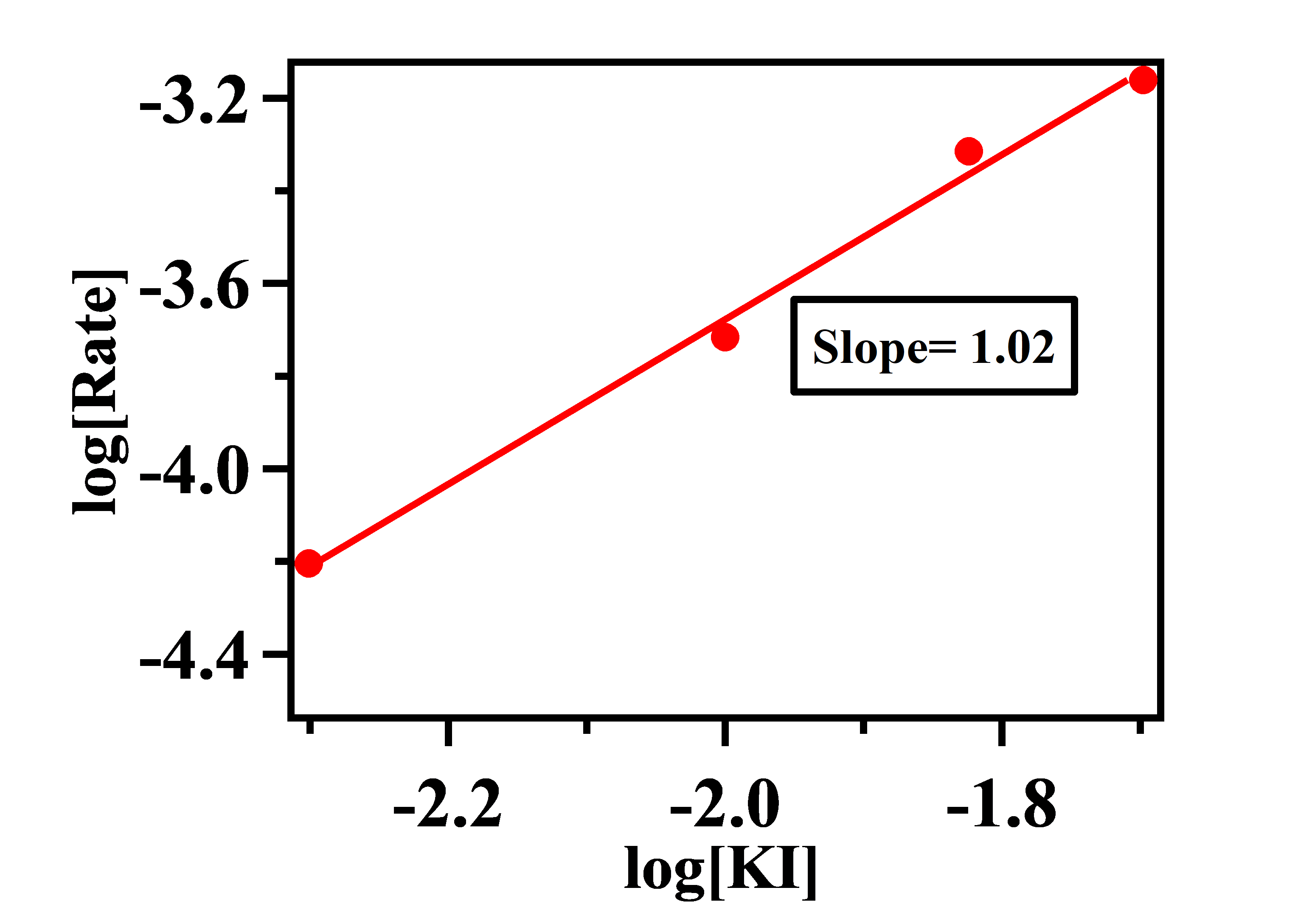
Here the order of the reaction with respect to KI = 1 (nearest possible integer of 1.02)
The rate law equation can be expressed as,
Rate = k [I–] [H2O2] , where ‘k’ is the rate constant.
Now, Rate=-(∆[H_2 O_2])/∆t , ∆[H_2 O_2]= 0.5× Moles of S2O32- consumed
= 0.5× 10 mL × 0.05 M × 0.001 mL-1
= 2.5 × 10-4 M
Rate=-(2.5 × 〖10〗^(-4) M)/(80 s) = 3.125 × 10-6 M.s-1
Rate constant, k = Rate/([I^-] [H_2 O_2]) = (3.125 × 〖10〗^(-6) M.s^(-1) )/(0.062 M×0.005 M) = 0.01 M-1 s-1
