Estimation of Iodine Value of Fats and Oils
Materials Required:
• Iodine Monochloride Reagent
• Potassium Iodide
• Standardized 0.1 N Sodium thiosulphate
• 1% Starch indicator solution
• Reagent bottle
• Chloroform
• Fat sample in chloroform
• Iodination flask
• Burette and burette stand with magnetic stirrer
• Glass pipette
• Measuring cylinder
• Distilled water
Method:
- Arrange all the reagent solutions prepared and the requirements on the table.
- Pipette out 10ml of fat sample dissolved in chloroform to an iodination flask labelled as “TEST".
- Add 20ml of Iodine Monochloride reagent in to the flask. Mix the contents in the flask thoroughly.
- Then the flask is allowed to stand for a half an hour incubation in dark.
- Set up a BLANK in another iodination flask by adding 10ml Chloroform to the flask.
- Add to the BLANK, 20ml of Iodine Monochloride reagent and mix the contents in the flask thoroughly.
- Incubate the BLANK in dark for 30 minutes.
- Meanwhile, Take out the TEST from incubation after 30 minutes and add 10 ml of potassium iodide solution into the flask.
- Rinse the stopper and the sides of the flask using 50 ml distilled water.
- Titrate the “TEST” against standardized sodium thiosulphate solution until a pale straw colour is observed.
- Add about 1ml starch indicator into the contents in the flask, a purple colour is observed.
- Continue the titration until the colour of the solution in the flask turns colourless.
- The disappearance of the blue colour is recorded as the end point of the titration.
- Similarly, the procedure is repeated for the flask labelled ‘Blank'.
Record the endpoint values of the BLANK.
Calculate the iodine number using the equation below:
Volume of Sodium thiosulphate used = [Blank- Test] ml
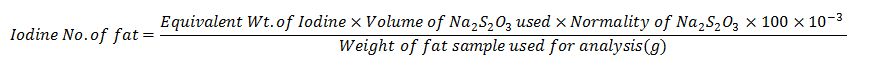
Equivalent Weight of Iodine = 127
Normality of sodium thiosulphate ( Na2S203) = 0.1
