Estimation of molecular weight from freezing point depression
Apparatus
A. Temperature-controlled magnetic stirrer
B. Thermometer
C. Beaker (500 mL)
D. Test tube
E. Naphthalene
F. Sulfur powder
G. Clamp
Procedure in laboratory (diagram)
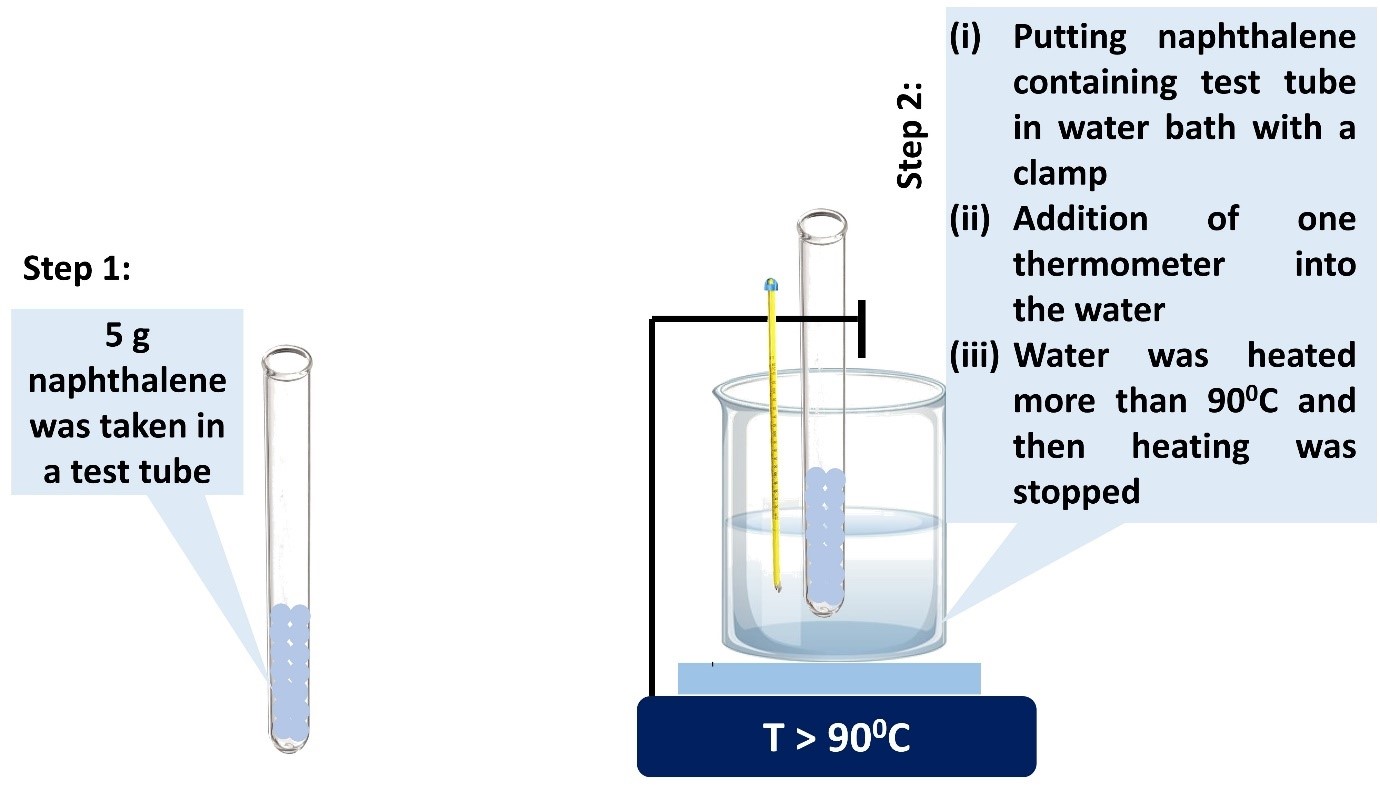
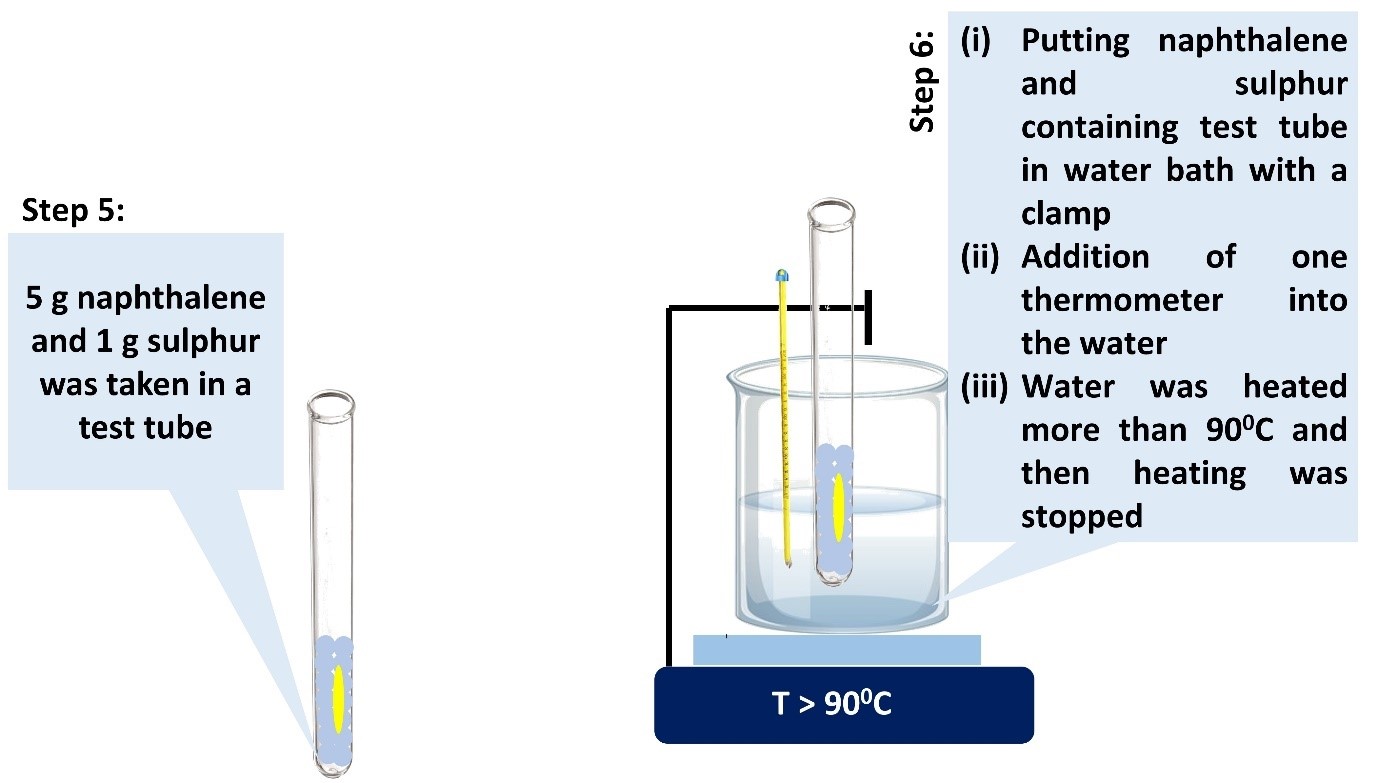
Depression of freezing point,
Procedure in laboratory (diagram)

Sample Data and Analysis
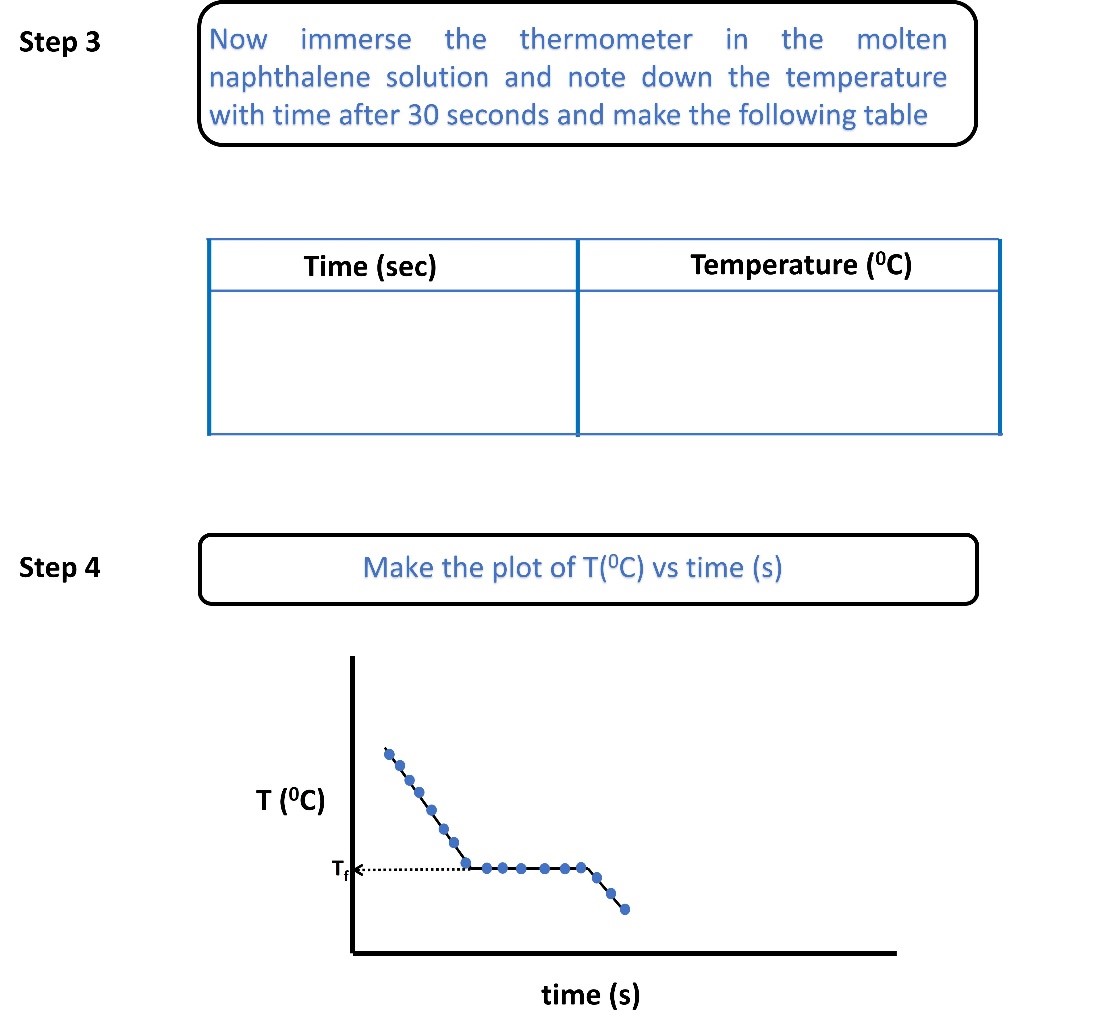
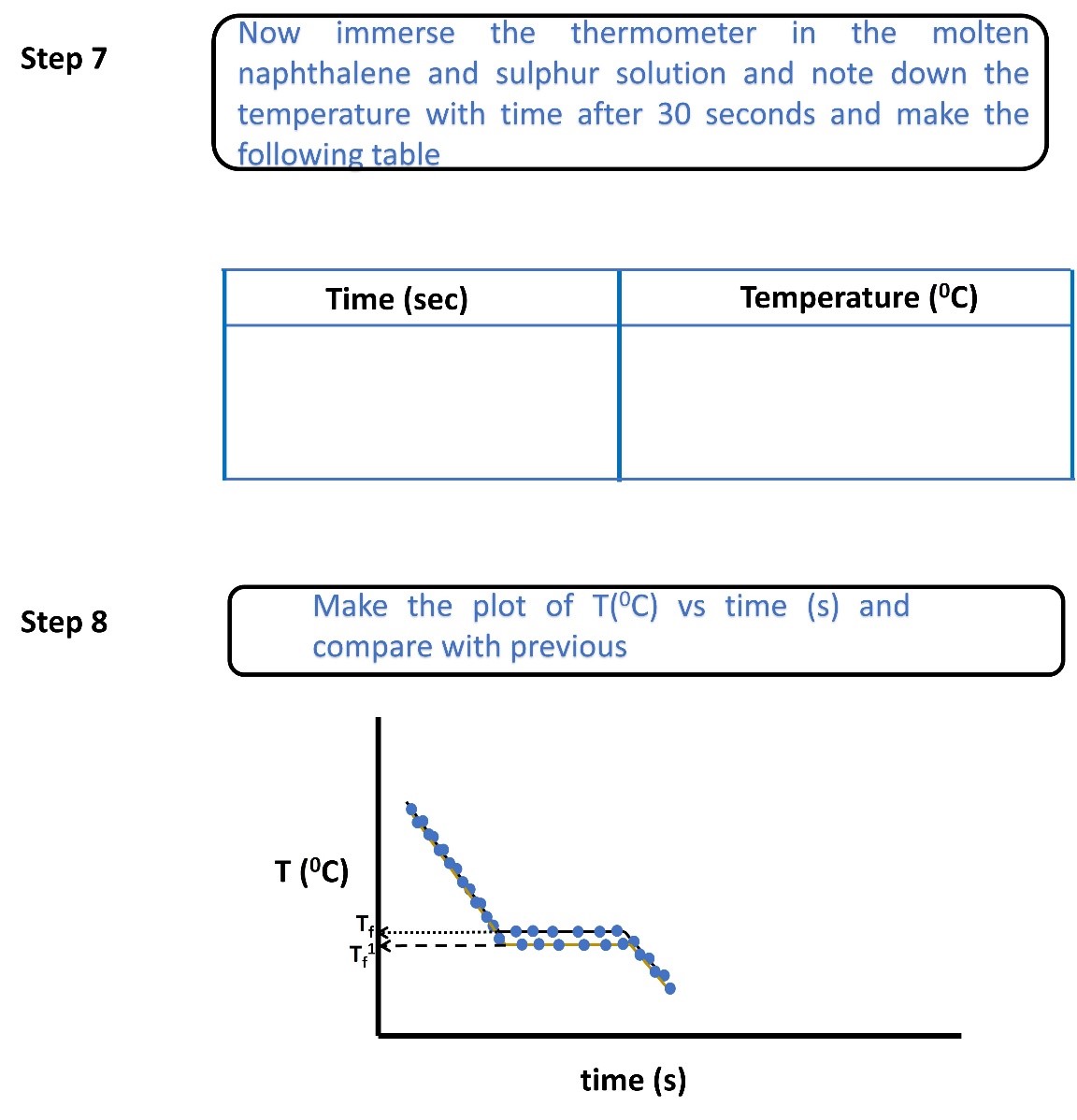
Determination of freezing point of pure naphthalene and naphthalene-solute mixture
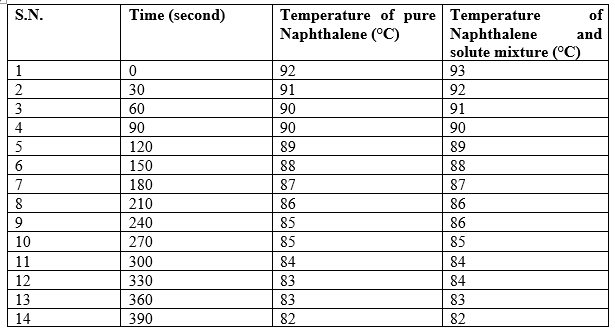
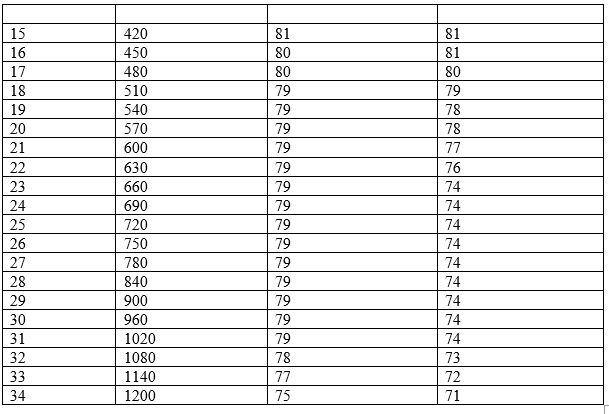
Calculation
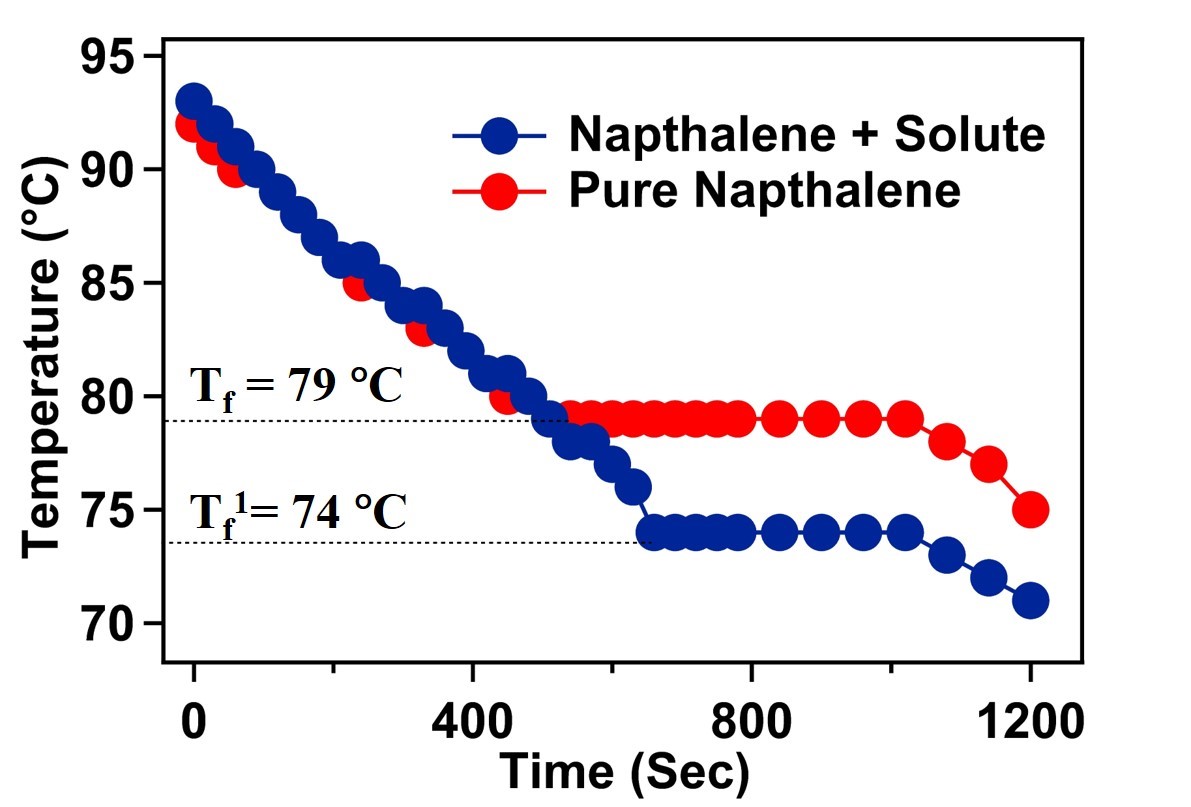
Figure 1. Temperature vs time graph of pure naphthalene and naphthalene-solute mixture.
The cryoscopic constant (K_f) of naphthalene is 6.9 °C/molality. From the experiment, we measured the depression in the freezing point (ΔT_f) of naphthalene after adding the unknown solute is 5 °C. For the experiment 5 gm (w_solvent)of naphthalene was taken and 1 gm (w_solute)of unknown solute was added in naphthalene. According to equation 5, the molecular weight of the solute added in the naphthalene solvent will be
M=Kf×wsolute×1000/ΔTf×wsolvent
M=(6.9×1×1000)/(5×5)=276 g mol(-1)
