Estimation of entropy and free energy change of a reaction
1) Apparatus :
A. Temperature controlled water bath.
B. Volumetric flask
C. Conical flask
D. Burette
E. Micropipette
F. Measuring cylinder
2) Procedure in laboratory (diagram) :
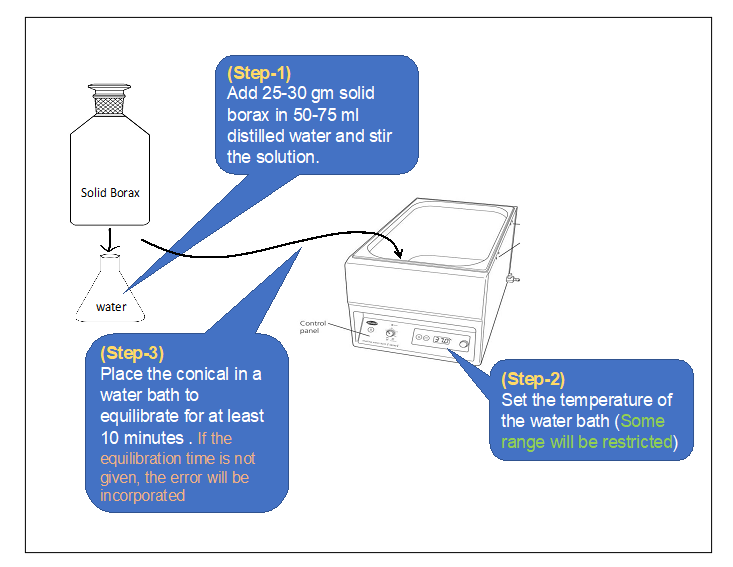
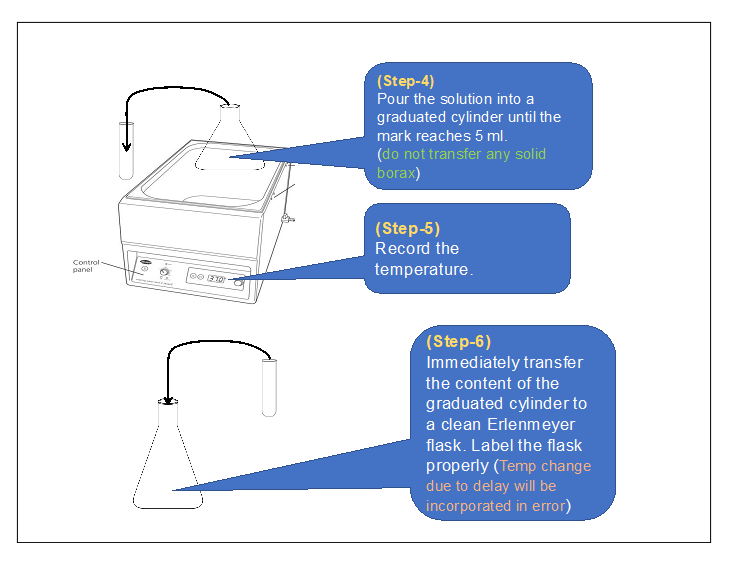
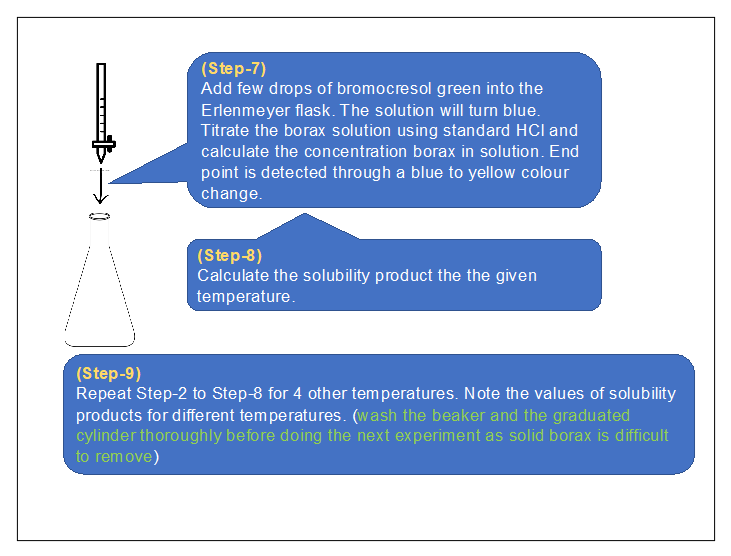
3) Procedure in laboratory :
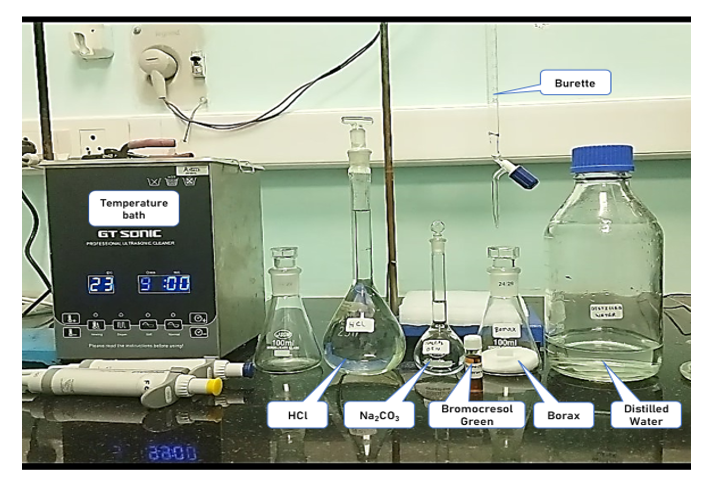
4) Data and the analysis :
4.1) Standardisation of HCl by Na2CO3 :
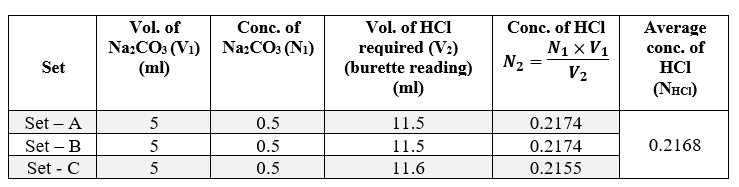
Therefore, the molarity of HCl (MHCl) = 0.2168 (M) [as, Normality ≡ Molarity for HCl]
4.2) Calculation of solubility product (ksp) of Borax :
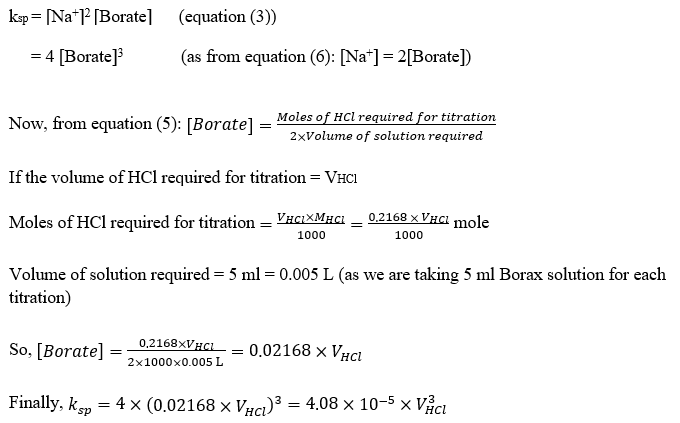
We will use the formula written above to calculate ksp at five different temperatures.
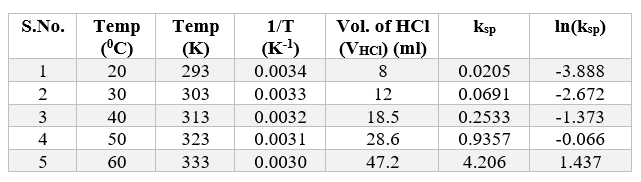
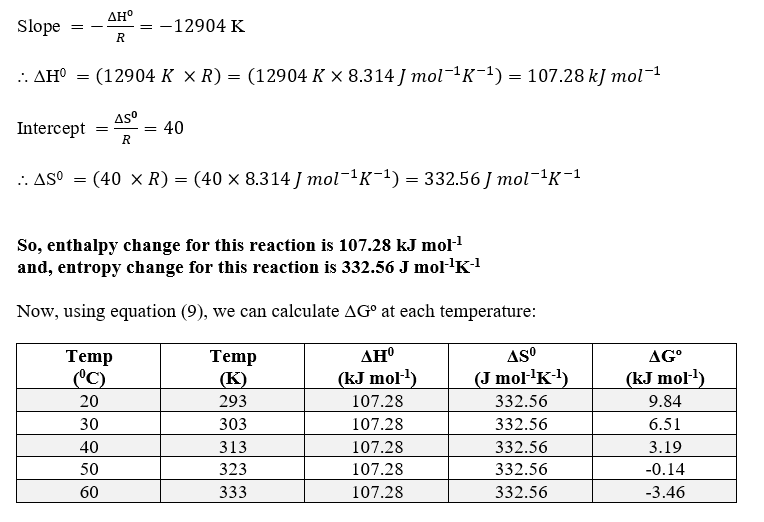
4.3) Plotting ln(ksp) vs 1/T to calculate ΔHº and ΔSº :
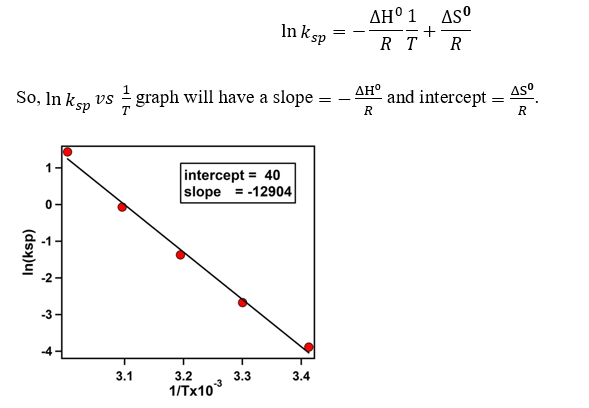
5) Analysis :