Conformational analysis of 1,2-dichloroethane
Among the butane conformers, when viewed about C 2 -C 3 bond, which occur at energy minima on a graph of potential energy versus dihedral angle?
The correct statement regarding conformation in butane is:
Select the correct statement concerning conformers of ethane and hexachloroethane:
Consider the potential energy diagram for rotation about the C2-C3 bond in pentane.The position marked A most likely corresponds to which of the following newmann projections.
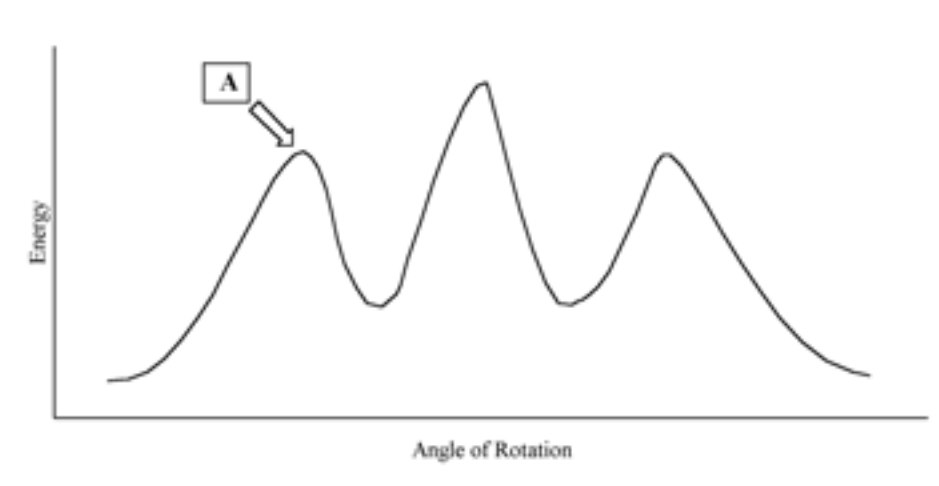

The most stable configuration of n-butane will be
