Instruction:
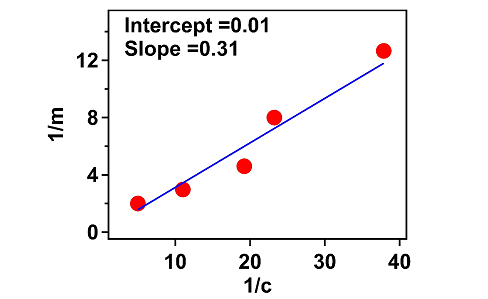
Figure 1: Plot of Langmuir adsorption isotherm at different concentrations of acetic acid From the graph, maximal substance amount of adsorbate (Amax) is calculated as and the value of adsorption constant (k) is calculated as
| S.N. | Volume of acetic acid (ml) | Initial reading of the burette (ml) | Final reading of the burette (ml) | Total volume of NaOH required (ml) | Strength of Acetic acid, C (N) |
|---|---|---|---|---|---|
| 1 | 10 | 0 | 41 | 41 | 0.197 |
| 2 | 10 | 0 | 18.9 | 18.9 | 0.091 |
| 3 | 10 | 0 | 10.9 | 10.9 | 0.052 |
| 4 | 10 | 0 | 8.9 | 8.9 | 0.043 |
| 5 | 10 | 0 | 5.5 | 5.5 | 0.026 |
| S.N. | Volume of acetic acid (ml) | Initial reading of the burette (ml) | Final reading of the burette (ml) | Total volume of NaOH required (ml) | Adsorbed acetic acid (mol/kg) |
|---|---|---|---|---|---|
| 1 | 10 | 0 | 38.5 | 38.5 | 0.5 |
| 2 | 10 | 0 | 15.2 | 15.2 | 0.336 |
| 3 | 10 | 0 | 6.7 | 6.7 | 0.22 |
| 4 | 10 | 0 | 6 | 6 | 0.125 |
| 5 | 10 | 0 | 2.5 | 2.5 | 0.08 |
| S.N. | Volume of NaOH before addition of charcoal (ml) | Volume of NaOH after addition of charcoal (ml) | Difference in Volume of NaOH | [AcOH] | Adsorbed acetic acid (mol/kg) (m) | 1/[AcOH] | 1/m |
|---|---|---|---|---|---|---|---|
| 1 | 41 | 38.5 | 0.197 | 5 | 2 | ||
| 2 | 18.9 | 15.2 | 0.091 | 11.02 | 2.98 | ||
| 3 | 10.9 | 6.7 | 0.052 | 19.23 | 4.55 | ||
| 4 | 8.9 | 6 | 0.043 | 23.25 | 8 | ||
| 5 | 5.5 | 2.5 | 0.026 | 37.88 | 12.66 |
| Difference in Volume of NaOH | Adsorbed acetic acid (mol/kg) (m) |
|---|---|
K1
K2
K3
K4
K5






.png)
.png)
.png)
.png)
.png)


| S.N. | Volume of acetic acid (ml) | Initial reading of the burette (ml) | Final reading of the burette (ml) | Total volume of NaOH required (ml) | Adsorbed acetic acid (mol/kg) |
|---|---|---|---|---|---|
| 1 | |||||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 |

K1
K2
K3
K4
K5












K1
K2
K3
K4
K5

























K1
K2
K3
K4
K5
















0.0gm
U1
U2
U3
U4
U5







.png)
.png)
.png)
.png)
.png)

| S.N. | Volume of acetic acid (ml) | Initial reading of the burette (ml) | Final reading of the burette (ml) | Total volume of NaOH required (ml) | Strength of Acetic acid, C (N) |
|---|---|---|---|---|---|
| 1 | |||||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 |


U1
U2
U3
U4
U5
















.png)
.png)
.png)

S1
S2
S3
| S.N. | Volume of oxalic acid (ml) | Volume of NaOH (ml) |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 |
S1
S2
S3








S1
S2
S3
Oxalic acid








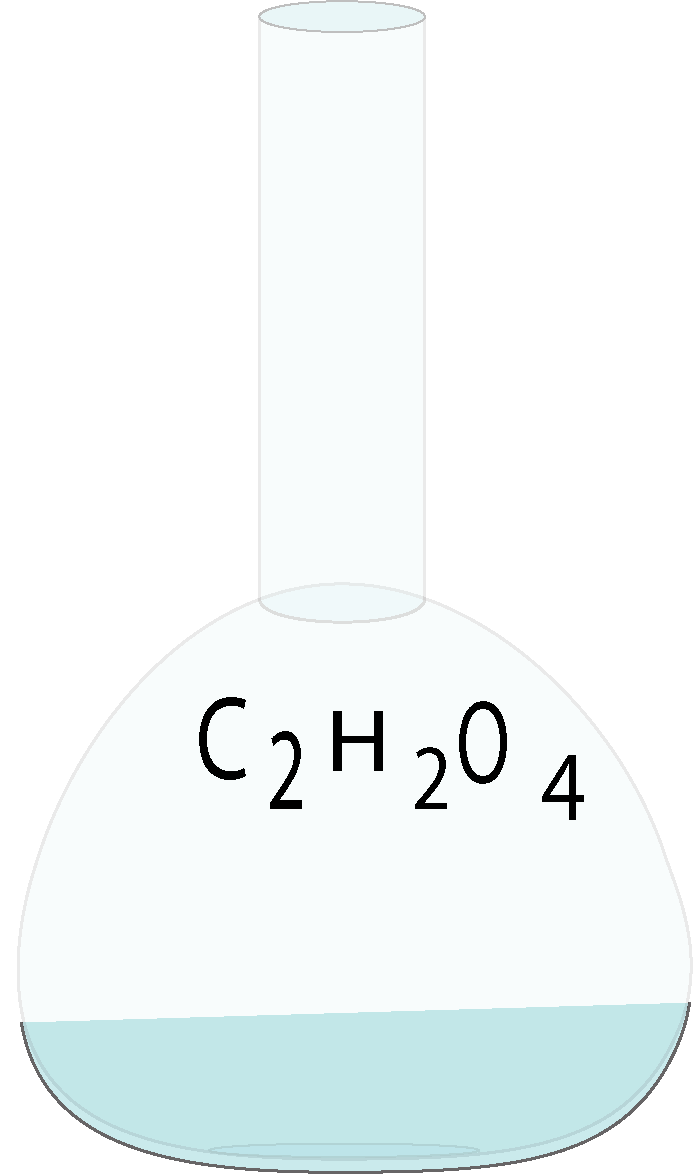
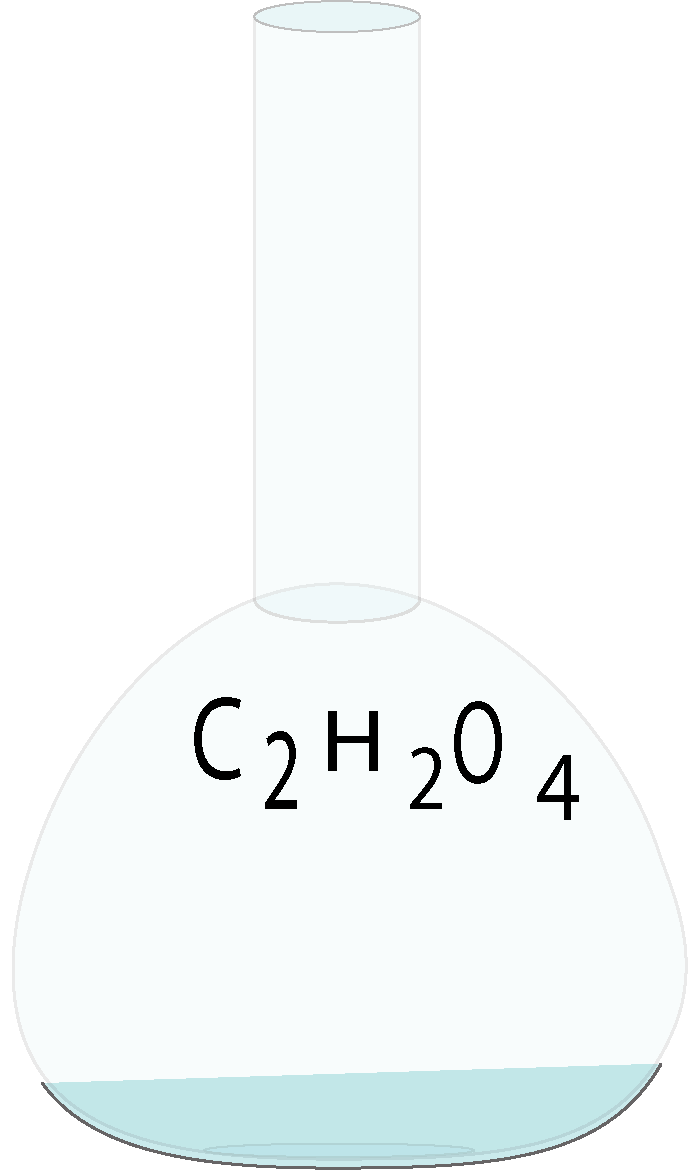
.png)
.png)
.png)
.png)





.png)

.png)









 `
`