Determination of Sulphate in Water
Objective:
To determine Sulphate ion concentration in sample by Turbidimetric method.
Apparatus used:
Spectrophotometer, Cuvettes, Pipette, Conical flasks, Magnetic stirrer, Spatula etc.
Solutions used Sulphate solution, Buffer solution, Barium chloride crystals, Distilled water, Water sample Description Sulphates is widely distributed in nature and may be present in natural waters in concentration ranging from few hundred to several thousand mg/l. Sulphates occur naturally in numerous minerals, including barite, epsomite and gypsum. These dissolved minerals contribute to the mineral content of drinking-water.




Preparation of Standard Solution
Prepare a series of standards of 100ml by accurately pipetting calculated volumes of standard Sulphate solution to measure 5 to 40 mg/l in conical flasks.






Press the up arrow on the bulb to take 5ml of Sulphate solution up into the pipette
Press the down arrow on the bulb to release Sulphate solutioninto the flask
Dilute the solution by adding distilled water
Prepare a blank solution and a sample solution by adding 100ml of distilled water and water sample to conical flasks respectively.








Press the up arrow on the bulb to take distilled water up into the pipette
Press the down arrow on the bulb to release distilled water into the flask
Dilute the solution by adding distilled water
Blank Solution
Sample Solution
Add 20ml of Buffer solution to conical flasks.







Press the up arrow on the bulb to take 20ml of Buffer solution up into the pipette
Press the down arrow on the bulb to release Buffer solutioninto the flask
Stir the solution in the flask using magnetic stirrer at constant speed.





Add a spatula of Barium Chloride crystals. Then stir the solution for 60-65 seconds and allow it to rest for 5 minutes.



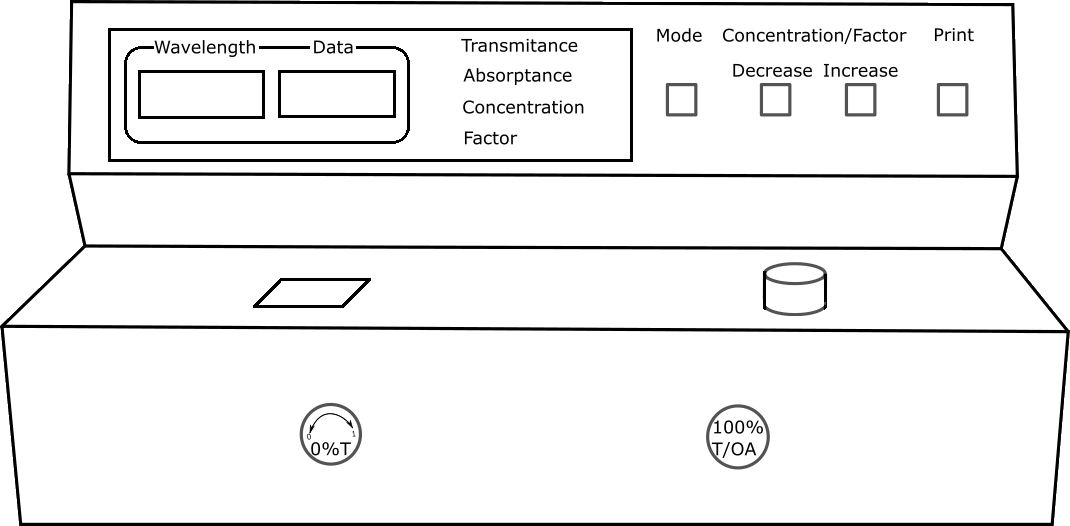
Calibration of Spectrophotometer
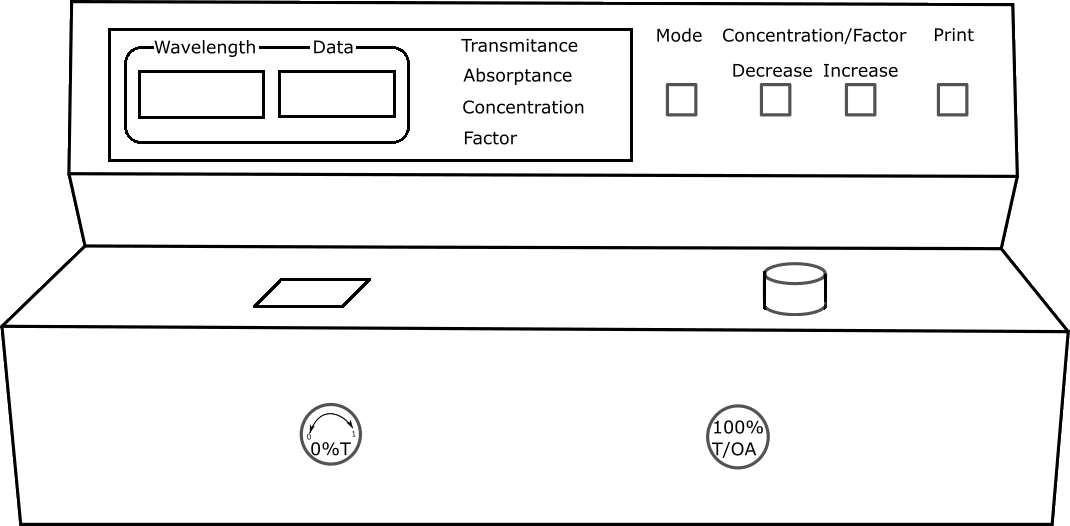
Fill the cuvette with blank solution and place it in Spectrophotometer. Set the wavelength to 420nm by adjusting wavelength control knob.














Measurement of Absorbance and Transmittance
Fill the cuvette with Sulphate solution in the flask. Place it in Spectrophotometer and note down absorbance and transmittance values for each solution.
Description Absorbance is the amount of light absorbed by a sample.It is calculated from T or %T using the following equations: A = - log10 T or A = log10 (1/T) A = 2 - log10 %T The amount of monochromatic light absorbed by a sample is determined by comparing the intensities of the incident light (Io) and transmitted light(I). The ratio of the intensity of the transmitted light (I) to the intensity of the incident light (Io) is called Transmittance(T).


















Observations:
| Sample | Sulphate concentration (mg/l) | Absorbance | Transmittance |
|---|
Characteristic Curves
Sulphate Concentration v/s Absorbance
Sulphate Concentration v/s Transmittance
Sulphate concentration of tap water = mg/l
Sulphate concentration of water sample = mg/l
✔
✔
✘
✘
Inference:
What is the acceptable range of Sulphate in drinking water?
0mg/l - 100mg/l 100mg/l - 150mg/l 150mg/l - 200mg/l 200mg/l - 250mg/l