Simulation
Reaction Kinetic Studies in Mixed Flow Reactor
Department of Chemical engineering NITK presents...
CONTINUE..
What would you like to do.
Reaction Kinetic Studies in Mixed Flow Reactor
VIRTUAL LAB @ NITK
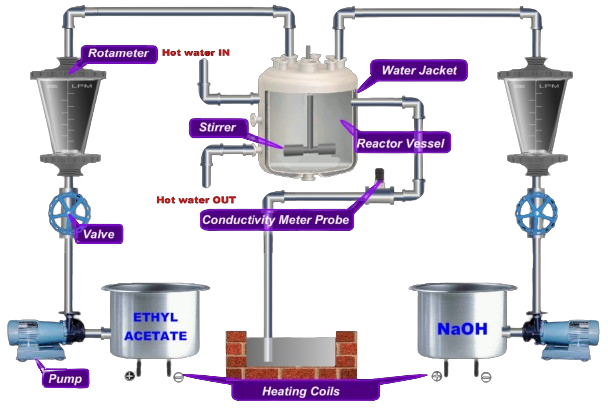
- Click on Label Tab to see the Experimental Setup.
- Click on Setup Tab to Configure Experimental Setup.
- Click on Experiment Tab to perform the experiment.
- Set NaOH Flowrate first and then Ethyl Acetate and wait till reactor is filled.
- Wait for reactor to achieve steady state and then add the readings to Observation .
- Click on Data tab to configure Experimental data.
- Click on Evaluate button to see the Results.
- Click on Evaluation tab to check the Simulated Values.
- To perform Experiment again click on title of experiment.
Reaction Temperature:
° Celsius
Rate Constant(k):
Litre/gmol.min
Frequency Factor(k°):
x108 (Litre/gmol.min)
Activation Energy:
J/mol
Reactor Volume:
Litre
Stock Concentration of Ethyl Acetate:
0.020 MStock Concentration of NaOH:
0.020 MReaction Temperature:
° Celsius
| Temperature (Celsius) |
Rate constant (litre/gmol.min) | Frequency Factor x10(-8)(litre/gmol.min) | Activation Energy(J/mol) |
|---|
| Temperature (Celsius) |
Rate constant (litre/gmol.min) | Frequency Factor x10(-8)(litre/gmol.min) | Activation Energy(J/mol) |
|---|
Experiment.













Rotameter Reading:
0.00 LPH
Rotameter Reading:
0.00 LPH
Hot water IN
Hot water OUT
ETHYL ACETATE
NaOH
| Process fluid | Manometric fluid | Flowrate(lpm) | h1(cm of Manometric fluid) | h2(cm of Manometric fluid) |
|---|

Observations
Click on the rows which you want to delete
| Ethyle Acetate Flowrate (Litre per Hour) |
NaOH Flowrate (Litre per Hour) |
Conductivity (milliSiemens/cm) |
|---|
Email is required
Experimental Data
Reactor Volume:
1000 Liter(s)
Stock Concentration of Ethyl Acetate:
0.020 M
Stock Concentration of NaOH:
0.020 M
Reaction Temperature:
30 degree Celsius
×
Manometric Fluid is about to Overflow.
Change the Manometer to Mercury.
Trial =