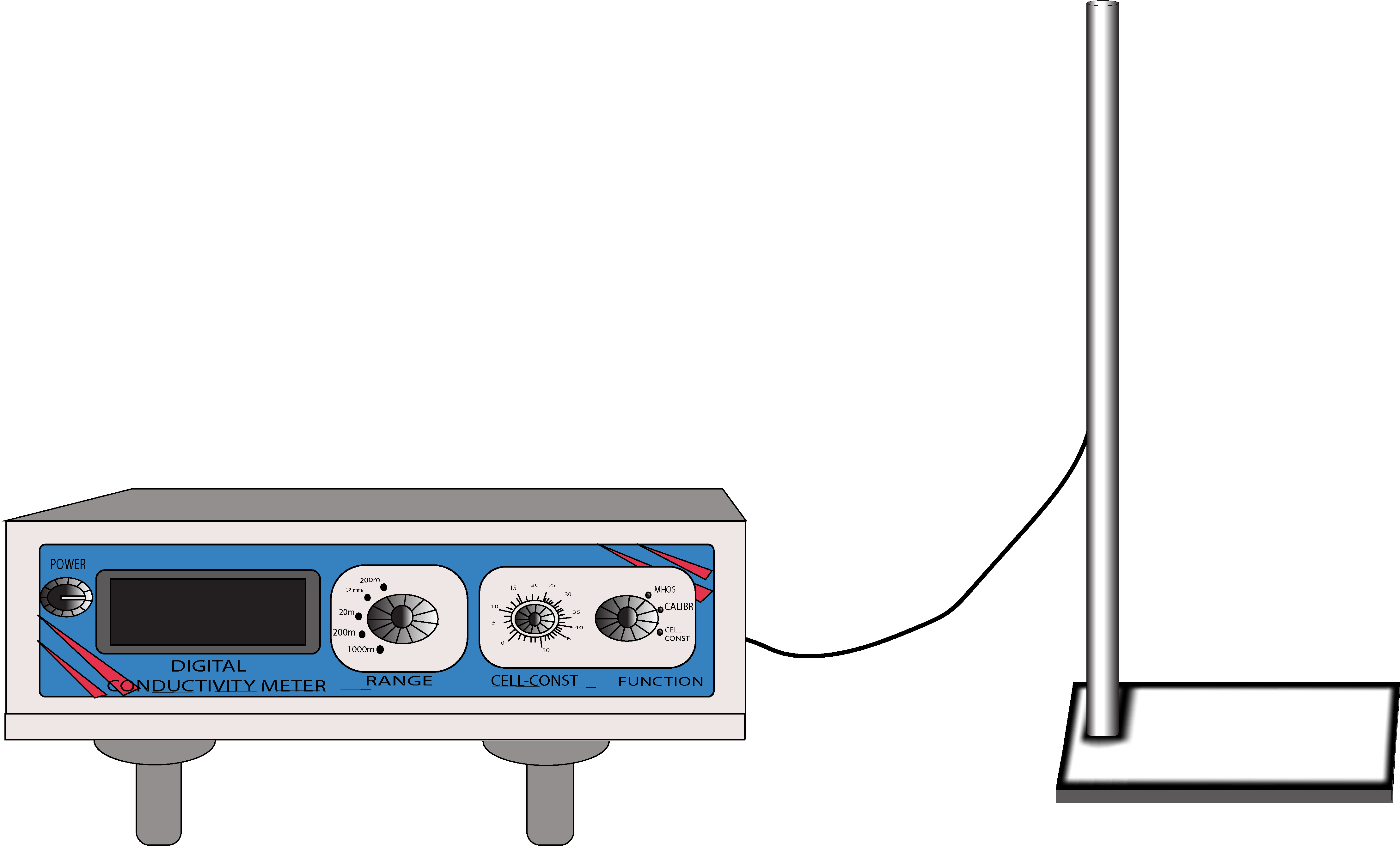
Measurement of Electrical conductance to determine dissociation constant of 'Acetic' acid





0.000




















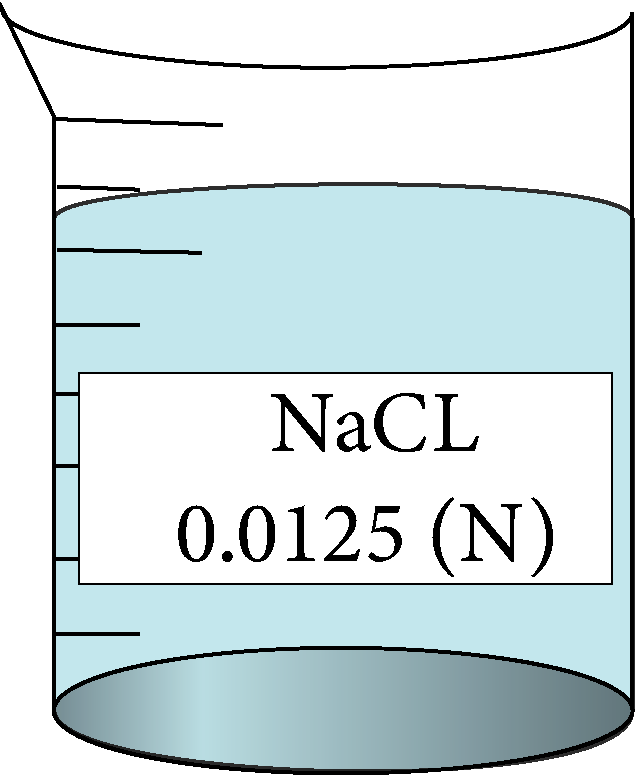



Remember
ok

Determination of equivalent conductance of NaCl at different concentrations
| Concentration of NaCl solution (N) |
√C(M0.5) | Conductance, L(mmho) |
Equivalent conductance, Λ (mmho cm2 equivalent-1) = (1000 L X)/C |
|---|---|---|---|
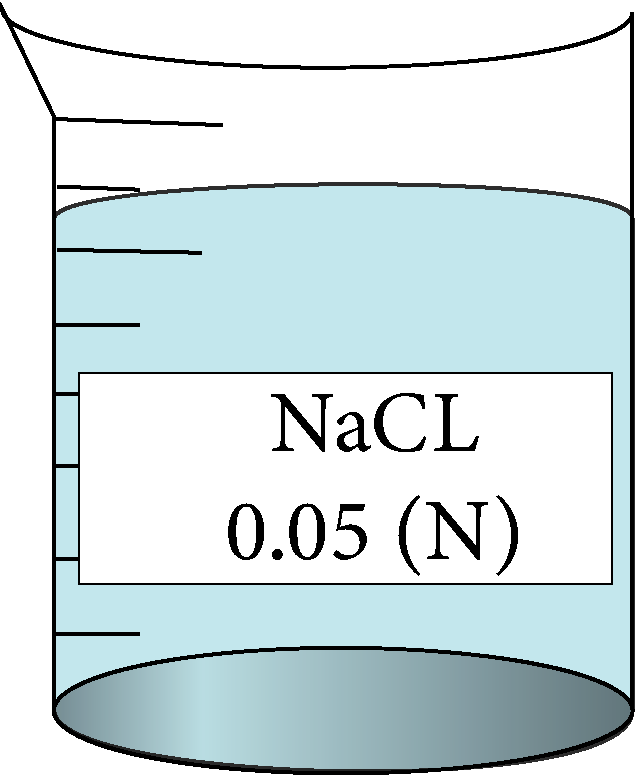
| 0.05 | 0.223607 | 5.95 | 138040 |
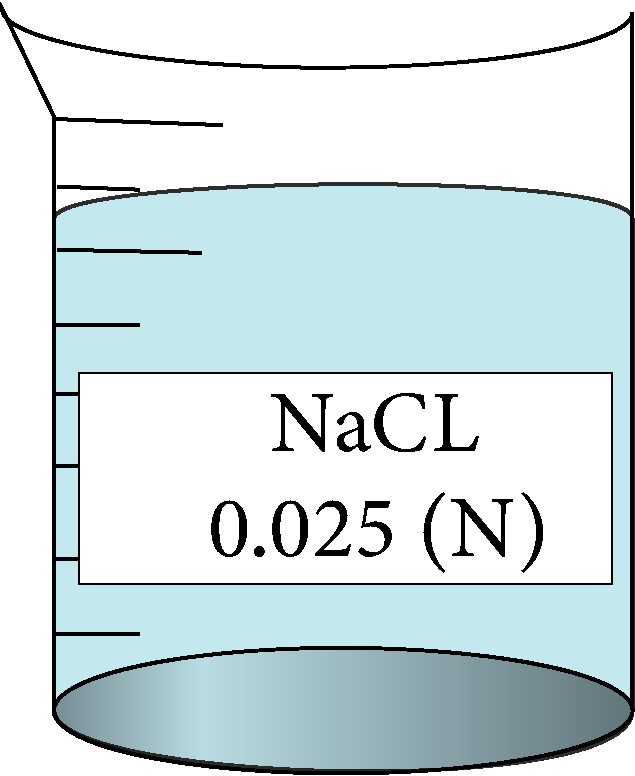
| 0.025 | 0.158114 | 2.77 | 128528 |
| 0.0125 | 0.111803 | 1.47 | 136416 |
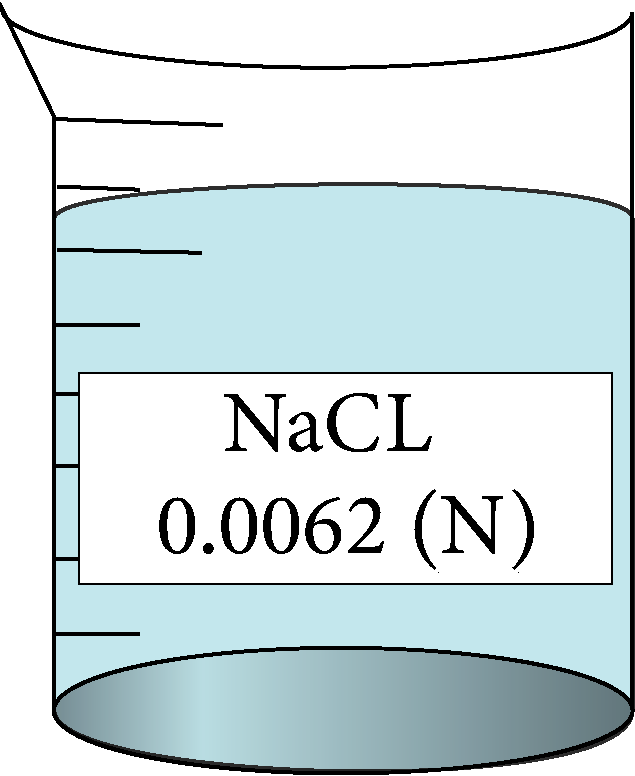
| 0.0062 | 0.0787401 | 0.77 | 144065 |
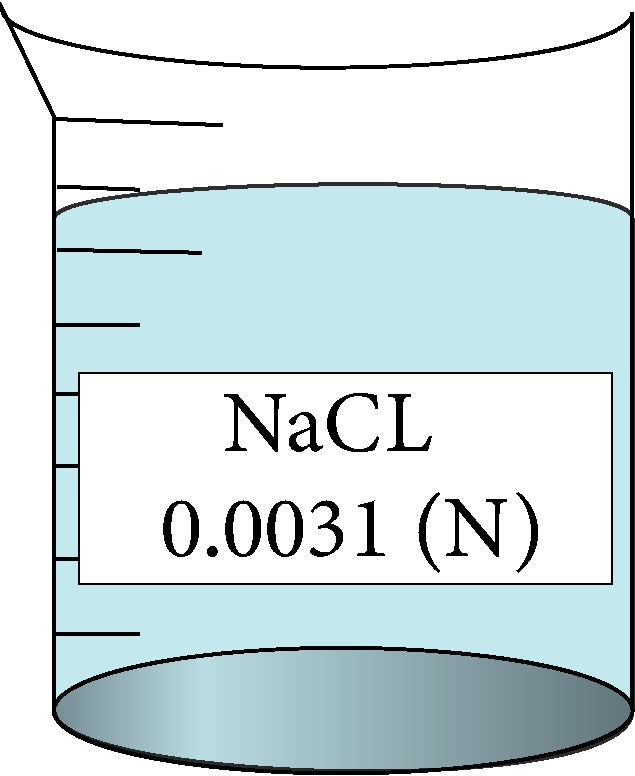
| 0.0031 | 0.0556776 | 0.41 | 153419 |
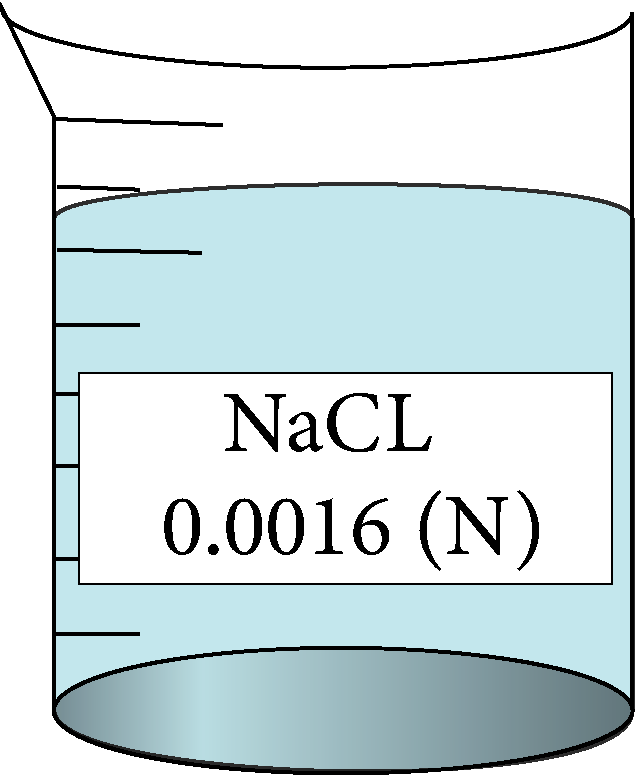
| 0.0016 | 0.04 | 0.23 | 166750 |
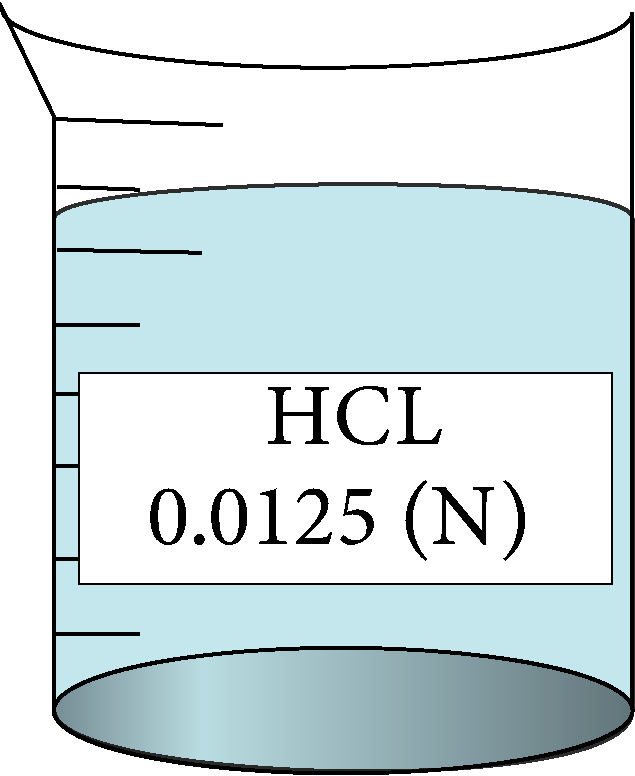
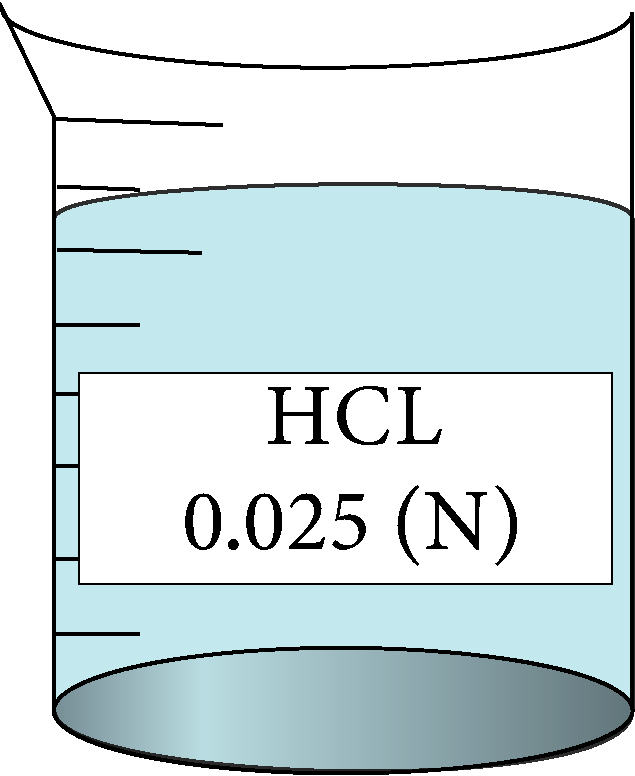
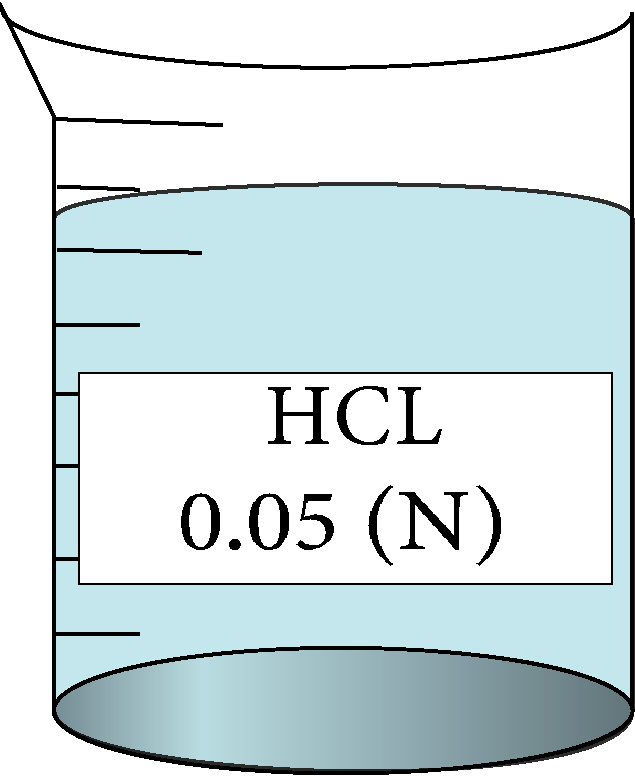
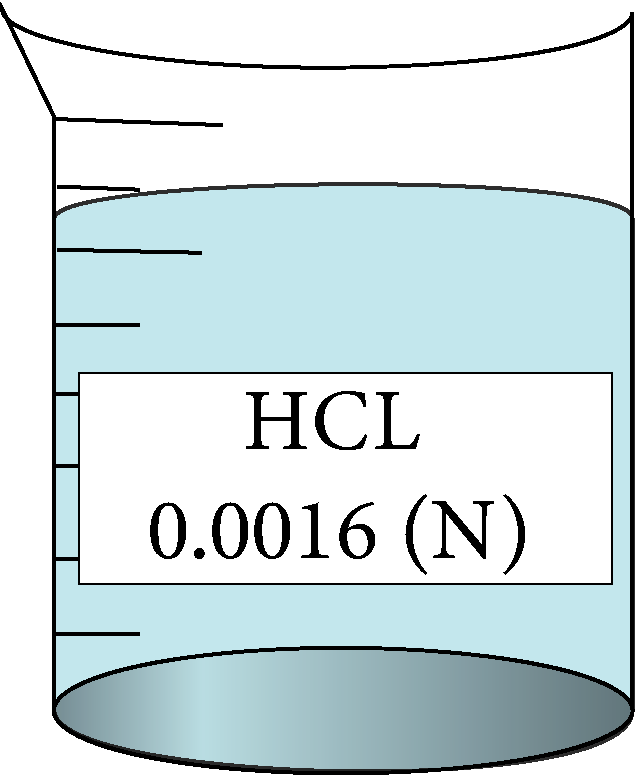
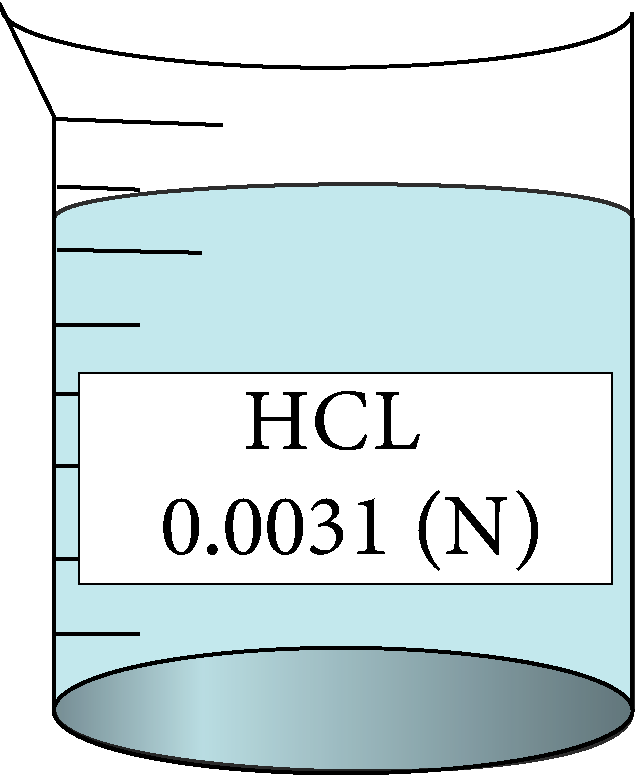
Determination of equivalent conductance of HCL at different concentrations
| Concentration of HCL solution (N) |
√C(M0.5) | Conductance, L(mmho) |
Equivalent conductance, Λ (mmho cm2 equivalent-1) = (1000 L X)/C |
|---|---|---|---|
| 0.05 | 0.223607 | 13.7 | 317840 |
| 0.025 | 0.158114 | 7.65 | 354960 |
| 0.0125 | 0.111803 | 4.15 | 385120 |
| 0.0062 | 0.0787401 | 2.14 | 400387 |
| 0.0031 | 0.0556776 | 1.12 | 419097 |
| 0.0016 | 0.04 | 0.6 | 435000 |
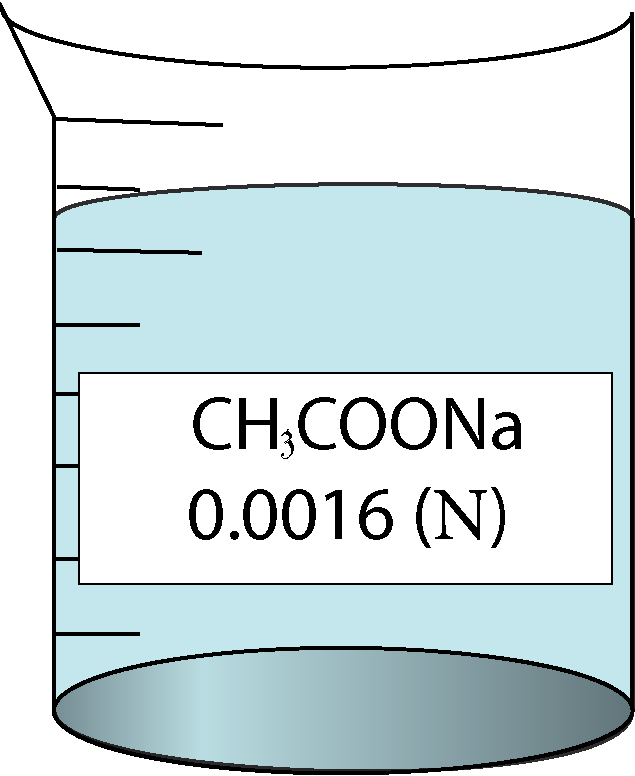
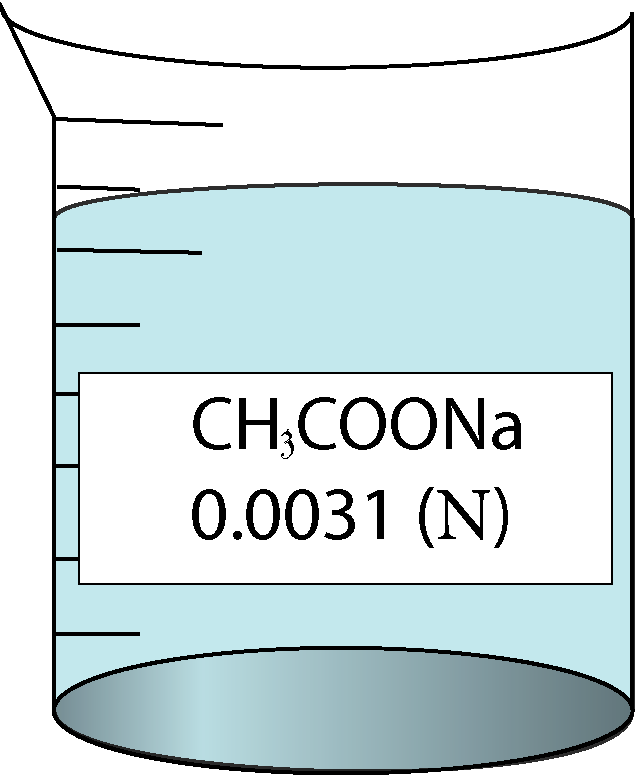
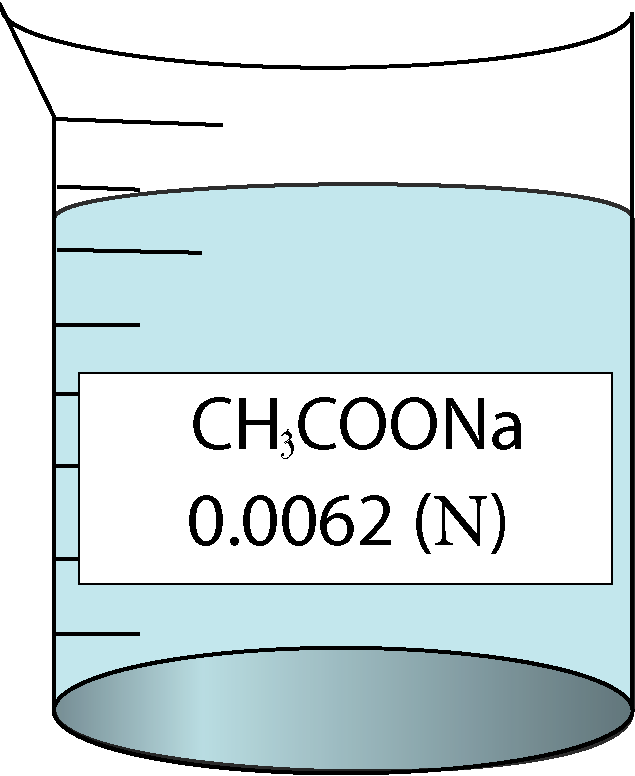
Determination of equivalent conductance of CH3COONa at different concentrations
| Concentration of CH3COONa solution (N) |
√C(M0.5) | Conductance, L(mmho) |
Equivalent conductance, Λ (mmho cm2 equivalent-1) = (1000 L X)/C |
|---|---|---|---|
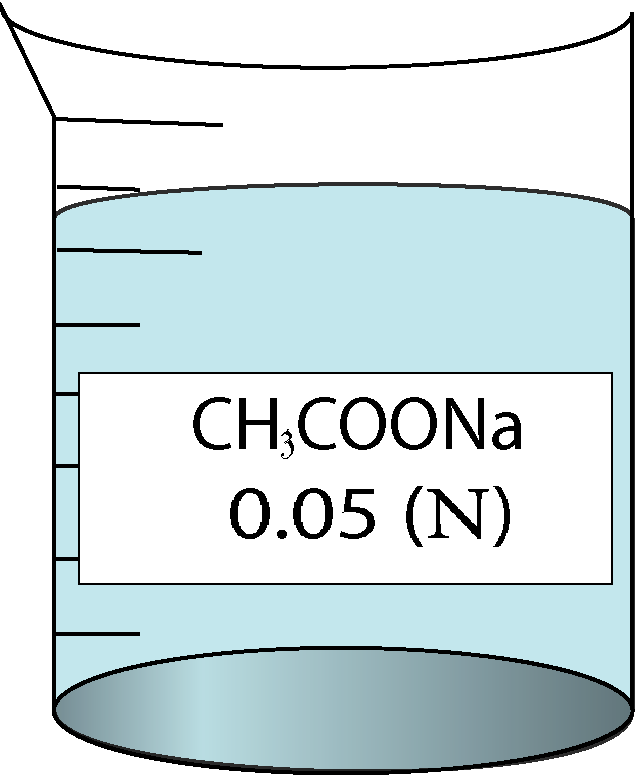
| 0.05 | 0.223607 | 2.26 | 52432 |
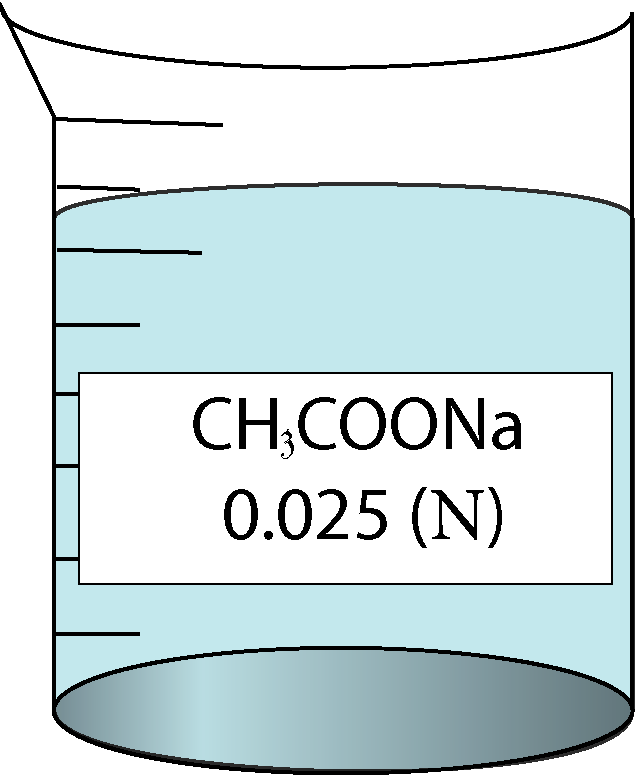
| 0.025 | 0.158114 | 1.46 | 67744 |
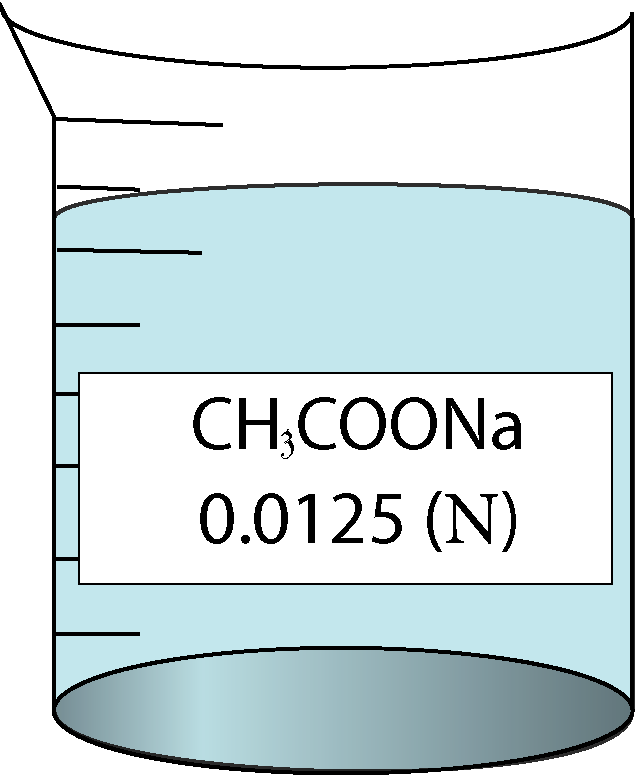
| 0.0125 | 0.111803 | 0.92 | 85376 |
| 0.0062 | 0.0787401 | 0.54 | 101032 |
| 0.0031 | 0.0556776 | 0.31 | 116000 |
| 0.0016 | 0.04 | 0.17 | 123250 |
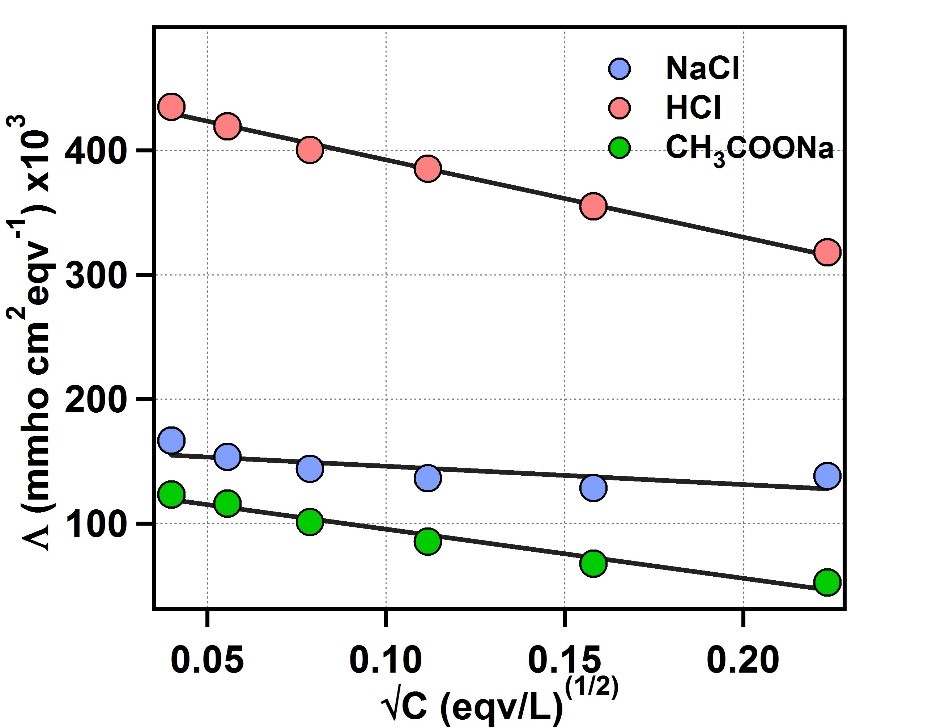
Figure 1: Plot of equivalent conductance vs √C for NaCl, HCl and CH3COONa
The Y-axis intercept for NaCl, HCl and CH3COONa are 169960 mmho cm2 eqv-1, 454680
mmho cm2 eqv-1, 135130 mmho cm2 eqv-1, respectively.
The equivalent conductance of acetic acid at infinite dilution (Λ0) is given as,
Λ0 = Λ0CH3COONa + Λ0HCl - Λ0NaCl
= (135130+454680-169960) mmho cm2 eqv-1
= 419850 mmho cm2 eqv-1
Determination of exact concentration of acetic acid solutions
| Approximate concentration of acetic acid solutions, C (N) |
Volume of standard NaOH required for titration (mL) |
Actual concentration of acetic acid solutions, C (N) |
|---|---|---|
| 1 | 9.1 | 0.91 |
| 0.5 | 4.8 | 0.48 |
| 0.25 | 2.4 | 0.24 |
Determination of dissociation constant of acetic acid by conductance measurement
| Exact concentration of acetic acid solutions, C (N) |
Conductance ,L (mmho) |
Equivalent Conductance, Λ (mmho cm2 eqv-1) = (1000LX)/C |
Equivalent conductance at infinite dilution of acetic acid, Λ0(mmho cm2 eqv-1) |
Degree of dissociation α = Λ/Λ0 |
Dissociation constant of acetic acid, Ka= (cα2)/(1-α) |
|---|---|---|---|---|---|
| 0.91 | 1.71 | 1873.85 | 419850 | 0.00446313 | 2.46569e-05 |
| 0.48 | 1.14 | 2755 | 0.00656187 | 2.08044e-05 | |
| 0.24 | 0.79 | 3818.33 | 0.00909452 | 2.00326e-05 | |
| 0.12 | 0.49 | 4736.67 | 0.0112818 | 1.54478e-05 | |
| 0.06 | 0.32 | 6186.67 | 0.0147354 | 1.32228e-05 |
Instructions
lets start the experiment
