Burette
Conical flask
Volumetric flask
Measuring Cylinder
Measuring Cylinder
Micro Pipette
Step0: Glassware & Chemical reagents required



Standardization of DCPIP Dye solution
Step1: Take 5 ml of standard ascorbic acid solution in the conical flask
click to suck ascorbic acid
click to pour in conical flasks
Step2: Add 5 ml of HPO3 solution in the conical flask
click to suck HPO3
click to pour in conical flasks
Step3: Titrate the mixture with DCPIP dye solution
click to place funnel
click to pour in dye
click to remove funnel
click to place flask
start titration
stop titration
| Sample | Titrant used |
|---|---|
| standard sample |
Titration Completed
Step4: Calculation of Dye factor
| Dye Factor = |
0.5
|
|---|---|
| Titrant used |
| Dye Factor = |
0.5
|
= 0.08 |
|---|---|---|
Analysis of sample
Step5: Selection of sample

select this sample

select this sample

select this sample

select this sample
Step6: Take out 50 ml juice in four different conical flasks marked as J1, J2, J3, J4
Fill J1
Fill J2
Fill J3
Fill J4
Step7: Heat the J2, J3, J4 sample at 60, 75, 90°C respectively for 10 minutes.
Turn on
Place J2
Place J3
Place J4
PT 00℃
ST 00℃

10:00
Step8: Pipette out 10 ml juice from each sample flask and pour to other four flask
Pipette out J1
Pipette out J2
Pipette out J3
Pipette out J4
Step9: Add 90 ml of 3% HPO3 solution in each conical flask.
Add in J1
Add in J2
Add in J3
Add in J4
Step10: Titrate each sample with DCPIP dye solution to a pink color which persists for 15s observation
Place funnel
Pour Dye
Remove funnel
Tirate J1
Tirate J2
Tirate J3
Tirate J4
start titration
stop titration
Titration Completed
| Sample | Titrant used |
|---|---|
| J1 | |
| J2 | |
| J3 | |
| J4 |
Calculation & Result
| Sample Name | Treatment | Titrant Used |
|---|---|---|
| J1 | Fresh | |
| J2 | Heated at 60C for 10 min | |
| J3 | Heated at 75C for 10 min | |
| J4 | Heated at 90C for 10 min |
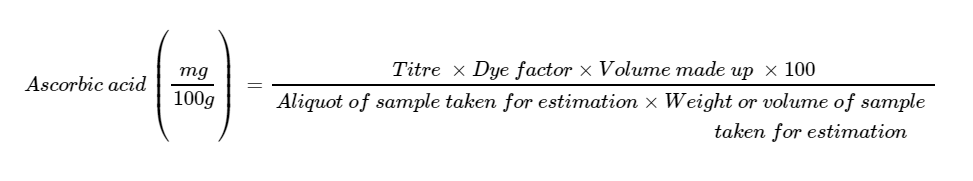
Ascorbic Acid Quiz
Round the calculation by 2 digits then check answers
Inference
The results indicated that the amount of ascorbic acid (Vitamin C)
decreases as the heating temperature of the sample increases.
It
shows that vitamin C is heat sensitive vitamin and degradated with
temperature.