Objective
To find the surface tension of kerosene by dropweight method.
Instructions
- Select the "Distilled water" from 'Select the experiment' dropdown menu.
- Find the weight of empty beaker in grams.
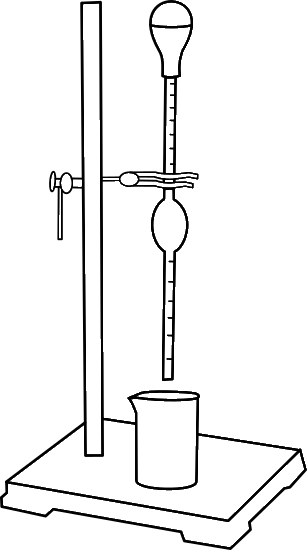
- Distilled water (reference liquid) is sucked into the stalagmometer (till point A) using a rubber bulb attached to the mouth of stalagmometer.
- Allow 20 drops of water to fall inside the beaker by clicking on the "OK" and again weigh the weight of the beaker with liquid.
- Click on the 'Observation' button to view the table.
- Change the procedure to "Kerosene" to calculate the dropping weight of kerosene.
- Click on the 'Start' button to start the experiment.
- Allow 20 drops of kerosene to fall inside the beaker and again weigh the weight of the beaker with liquid.
- Click on the 'Observation' button to view the table.
- Click on the 'Show Result' button to view the result.











Coefficient of Viscosity of
,η=00