Determination of Total Iron in water
Objective:
To determine concentration of total iron content in water sample by Spectrophotometric method.
Apparatus used:
Nessler tubes, Spectrophotometer, Cuvettes, Graduated pipette etc.
Solutions used Standard iron solution, Dilute hydrochloric acid, Distilled water, Water sample, Potassium Thiocyanate solution Description Iron can exist in water in several different forms:- Ferrous iron (Fe2+)
- Ferric iron (Fe3+)
- Complexed iron, in which iron is bound with various chemicals such as EDTA
- Iron oxides, such as rust




Preparation of Standard Solution
Prepare a series of standards of 100ml by accurately pipetting calculated volumes of standard Iron solution to measure 0 to 2.5mg/l in 100ml Nessler tube.






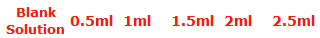





Take 2ml of tap water and 2ml of water sample in separate Nessler tubes.







Press the up arrow on the bulb to take solution up into the pipette
Press the down arrow on the bulb to release solution into the Nessler tube
Dilute the solution by adding distilled water
Tap Water
Sample Solution
Add 4ml dilute Hydrochloric Acid to all the Nessler tubes.






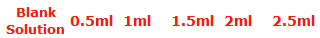


HydrochloricAcid



Add 5ml of Potassium Thiocyanate solution to all the Nessler tubes. Then add distilled water to the Nessler tubes upto the mark of 100ml.






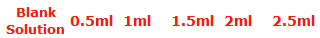







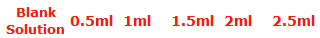
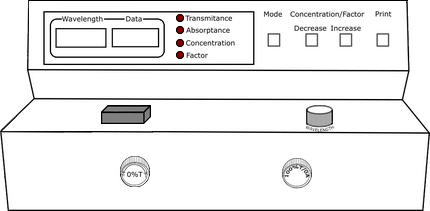
Calibration of Spectrophotometer
Fill the cuvette with blank solution and place it in spectrophotometer. Set the wavelength to 510nm by adjusting wavelength control knob.



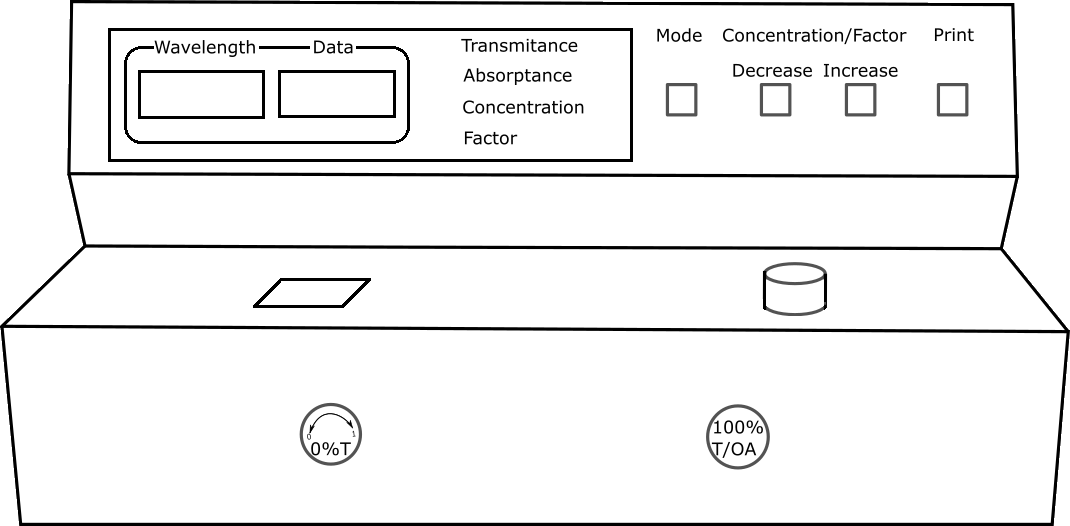





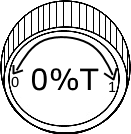



 Note
Note
- Setting condition is important for using the actual suitable curve
- Setting can be done in both absorbance and transmittance
- For blank sample, transmittance is 100 and absorbance is 0
Meassurement of Absorbance and Transmittance
Fill the cuvette with sulphate solution in the Nessler tube. Place it in spectrophotometer and note down absorbance and transmittance values for each solution.

















Observations:
| Sample | Iron content (mg/l) | Absorbance | Transmittance |
|---|
Characteristic Curves
Iron concentration v/s Absorbance
Iron concentration v/s Transmittance
Iron concentration of water sample = mg/l
Iron concentration of tap water = mg/l
✔
✔
✘
✘
Inference:
The acceptable range of iron in drinking water is >0.3mg/l.
True False