Determination of Biological Oxygen Demand
Objective:
To find the Biological Oxygen Demand exerted by the given sample of waste water.
Apparatus used:
BOD bottles, Pipettes, Conical flask, Burette, Beaker, Dropper, BOD incubator etc.
Solutions used Manganese Sulphate solution, Alkali Iodide Azide solution, Conc. Sulphuric acid, Sodium Thiosulphate solution, Starch indicator.




 Description
Biological Oxygen Demand or BOD is the amount of oxygen consumed by the organisms in breaking down the waste.
Description
Biological Oxygen Demand or BOD is the amount of oxygen consumed by the organisms in breaking down the waste.
Take 200ml of sample in 2 BOD bottles and dilute it with Dilution water by filling the water up to bottle neck.






















Add 1ml of Manganese Sulphate (MnSO4) solution to bottle named "Sample A".













Add 1 ml of Alkali-Iodide-Azide solution to Sample A bottle.













Mix the solution by inverting BOD bottle upside-down for 25-30 times and allow the precipitate to settle down at the bottom.








Allow the precipitate to settle down for few minutes
If there is no DO present, then white precipitate will form.
Add 2ml of concentrated H2SO4 carefully without forming air bubbles.











Squeeze the pipette bulb and dip pipette into the H2SO4 solution
Press the up arrow on the bulb to take the liquid up into the pipette
Mix the solution by inverting the BOD bottle till all the precipitate dissolves.








Take 200ml of sample in a conical flask using pipette.
























For calculation purpose volume of sample is considered as 200ml
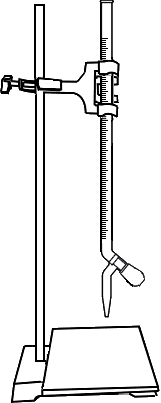
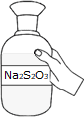
Take 0.025N Sodium Thiosulphate (Na2S2O3) solution in the burette.



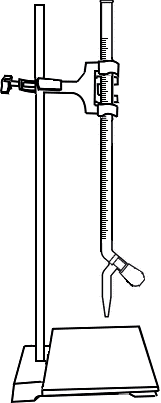



Titrate the solution against Na2S2O3. Add Starch when the colour of the solution changes to pale yellow. Then continue the titrarion till blue colour becomes colourless.









Open the knob to start the liquid running into the conical flask
Close the knob when the colour of solution in conical flask changes to pale yellow
Add 1ml Starch into conical flask
Initial burette reading = 0 ml
Observations:
| Volume of the sample (ml) | Burette Reading (ml) | Volume of Na2S2O3 ( Final Value - Initial Value ) (ml) | |
| Initial | Final | ||
| 200 | |||
Keep "Sample B" in the BOD incubator for 5 days at 20°C.


°C
15
Set the temperature to 20°C
After 5 days




Add 1ml of MnSO4 solution to BOD bottle named as Sample B.













Add 1ml of Alkali-Iodide-Azide solution to Sample B.













Mix the solution by inverting BOD bottle upside-down for 25-30 times and allow the precipitate to settle down at the bottom.









Allow the precipitate to settle down for few minutes
Add 2ml of concentrated H2SO4 carefully without forming air bubbles.



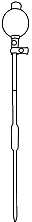









Squeeze the pipette bulb and dip pipette into the H2SO4 solution
Press the up arrow on the bulb to take the liquid up into the pipette
Mix the solution by inverting till all the precipitate dissolves.









Take 200ml of sample in a conical flask using pipette.

























For calculation purpose volume of sample is considered as 200ml
Take Sodium Thiosulphate (Na2S2O3) solution in the burette.








Titrate the solution against 0.025N Na2S2O3. Add Starch when the colour of the solution changes to pale yellow. Then continue the titrarion till blue colour becomes colourless.












Open the knob to start the liquid running into the conical flask
Close the knob when the colour of solution in conical flask changes to pale yellow
Add 1ml Starch into conical flask
Initial burette reading = 0 ml
Final burette reading = 6.4 ml
Observations:
| Sample | Volume of the sample (ml) | Burette Reading (ml) | Volume of Na2S2O3 ( Final Value - Initial Value ) (ml) | |
| Initial | Final | |||
| A | 200 | |||
| B | 200 | |||
✔
✘
✔
✘
✔
✘
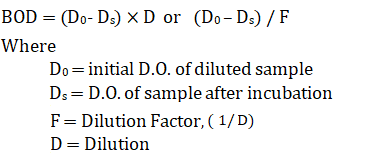
Inference:
What is the acceptable range of Biological Oxygen Demand in drinking water?
< 6mg/l 6mg/l - 30mg/l >30mg/l Cannot predictTitration =